We are working our way through each of the eighteen Groups of the Periodic Table (a.k.a. the Periodic Table of the Elements), with the general idea behind this series being to ensure that at least one element is included from each group.
So far, we’ve considered elements from eleven of these:
- Group 1: Hydrogen, and Sodium
- Group 2: Calcium
- Group 4: Titanium
- Group 8: Iron
- Group 10: Platinum
- Group 11: Copper
- Group 12: Mercury
- Group 13: Boron
- Group 14: Carbon
- Group 16: Oxygen, and Sulphur
- Group 17: Bromine
In this episode, we’re going to look at the 27th element in the table, one which falls into Group 9. Our element on this occasion can achieve, under certain circumstances (which will become apparent later), a serious case of the blues. It’s the metal cobalt.
2012rc, licensed under CC BY 3.0
A transition metal, cobalt sits at the top of Group 9 of the periodic table. Its stablemates are rhodium (Rh), iridium (Ir), and meitnerium (Mt). Rhodium and iridium are both hard silvery-white metals with high melting points, quite similar to cobalt in many ways. They are some of the rarest elements on earth. To date, meitnerium is still something of a mystery. A manmade element first synthesised in 1982, precious little is known of its properties. This isn’t too surprising as, being fiercely radioactive, it doesn’t hang about long for anyone to find out —even its most stable isotope, 278Mt, has a half-life of just four and a half seconds.
Our element, cobalt, is a moderately reactive metal. It reacts slowly with dilute acids, and will react with carbon, sulphur, and phosphorus at high temperatures, but does not react directly with either hydrogen or nitrogen.
Cobalt will, if heated, react with oxygen, including the oxygen found in air and water vapour. As we saw with titanium, cobalt is an element that forms a passive oxide layer which binds to the surface of the metal. Thus, although larger chunks of cobalt metal are relatively inert in air, at temperatures above 300 °C (570 °F) extensive oxidation will occur. In fact, even at lower temperatures it will react with moist air, just very slowly. However, despite not being greatly reactive, finely ground cobalt, in powdered form, can ignite spontaneously.
Cobalt usually donates its outermost electrons to form compounds—there are two of these, but our element actually has nine valence electrons that can participate in the formation of a chemical bond. The most common and most stable oxidation state for cobalt is +2. However, there are +3 oxidation state compounds which are also stable. Theoretically, it is possible for cobalt to achieve oxidation states of −3, −1, 0, +1, +2, +3, +4, and +5.
Considering cobalt as a pure metal, it is lustrous, and silvery-blue to steel-grey in colour. It is a moderately dense metal, similar in density to both copper and iron. It’s fairly hard (at 5 on the Mohs scale), and brittle. It is ductile, but only somewhat malleable. It has a high melting point (1,768 Kelvin, 1,495 °C).

Alchemist-hp, licensed under CC BY-SA 3.0
In comparison with the electrical conductivity of silver and copper, the best of all the elements, cobalt is not a fantastic conductor. However, it is plenty good enough that it still finds uses in batteries (primarily lithium-ion batteries), and in electronic equipment.
Sitting as it does, between the other ferromagnetic ‘d block’ transition metals iron and nickel at the top of the periodic table, cobalt possesses excellent magnetic properties. It actually maintains its ferromagnetic properties at high temperatures, up to 1,121 °C (2,050 °F), giving cobalt an advantage where magnetic properties are required at elevated temperatures. Furthermore, when cobalt is alloyed (combined with another metal, e.g., aluminium or nickel) its magnetic properties are strengthened.
In metallic form, cobalt has two distinct allotropes. The one which is (nominally) most common and stable at room temperature, in fact at temperatures below 417 °C, is the beta form (β-cobalt). This has a closely packed hexagonal, crystalline structure. At higher temperatures, the alpha form (α-cobalt) predominates. This form exhibits a simple face-centred cubic structure. Although this sounds straightforward, in fact elemental cobalt tends to exist as a mixture of the two allotropes over a broad temperature range, as the conversion between the α- and β- allotropes is extremely slow.
Cobalt has just a single naturally occurring isotope, 59Co, and this is stable. There are a further twenty-eight radioisotopes of cobalt currently known, which range from 47Co to 75Co, but none of these are naturally occurring, they are all synthetic isotopes produced by nuclear reactions.
To date, only two of cobalt’s radioisotopes, 57Co and 60Co, have found practical applications. Of these, 57Co is relatively short-lived, with a half-life of between 270 and 272 days. It is used in nuclear medicine as a radiolabel to help detect cancer, and also to determine how effectively an individual can absorb vitamin B12. More of this later.
However, 60Co persists for much longer, with a half-life of 5.27 years. This radioisotope is deliberately manufactured, produced in reactors in Argentina, Canada, China, India, and Russia. It is a high-intensity emitter of gamma-rays, therefore the energy produced is highly penetrating, which has earned it a place in in radiotherapy as one avenue of cancer treatments.
This radioisotope has also been exploited for a range of alternative uses and is, in fact, the largest revenue-generating commercial radioisotope in the world. 60Co is used in the inspection of materials, to gauge levelness or thickness, or to reveal the internal structure, including the presence of any flaws, or foreign objects. In similar fashion, 60Co scanners have been trialled to detect smuggled goods (e.g., illicit drugs and explosives) in cargo and airfreight containers. This has not been without its problems, however.
It is one of the radioisotopes used in food irradiation, a controversial technique whereby foodstuffs are exposed to the ionising radiation produced in the form of gamma-rays. This destroys microorganisms (and larger pests) responsible for food spoilage, but also inhibits the ripening and sprouting process of food in storage, with both aspects acting to extend product shelf-life. It is worth noting that the World Health Organization (WHO) has recommended its use, but I realised that I had no idea how one might identify foods which had been so treated. It appears that there is an internationally recognised symbol to indicate this, the ‘Radura’ symbol. One to look out for perhaps?
One final gem when it comes to the 60Co radioisotope is that it has been discussed as a means of producing an extremely ‘dirty’ bomb from which enhanced fall-out would render large areas completely uninhabitable for decades. This relates to ‘salting’ nuclear weapons, a diabolical concept I briefly discussed in a previous article, covering the element sodium.
By encasing a thermonuclear bomb in bog-standard cobalt metal (59Co), the explosion would convert the 59Co to radioactive 60Co by nuclear fusion. The resulting small radioactive particles would be airborne, so dispersed extensively by the explosion, falling back to earth over a wide area, likely extended via prevailing winds. Though this technology has ‘apparently’ not yet been developed in practical terms, speculation about the dreadfulness of such a ‘doomsday’ device has been part of popular culture since the late 1950s, when Nevil Shute made mention of ‘cobalt bombs’ in his novel On the Beach.
Incidentally, we Brits were the first to include cobalt in a nuclear device, albeit without the intention of deliberately ‘salting’ the device. One of a number of nuclear tests conducted in 1957 at the Maralinga site in South Australia was Operation Antler’s 1 kiloton ‘Tadje’ tower-detonated (atmospheric) test. This was part of trials of new lightweight plutonium nuclear weapons. The ‘Tadje’ test included cobalt pellets to act as a tracer so that yield could be determined.
As a small aside, in these heady days of exploration and a desire to be one step ahead in the Cold War, over 22,000 British servicemen, together with AWRE scientists and civilians were part of the nuclear tests. Some were used directly to monitor the effects of these explosions, and most often the men were provided with no protective equipment at all. In the heat of Maralinga, boots, a pair of shorts and maybe a hat would have been typical attire. Exposure to all kinds of nasties, not least radiation, was commonplace. Because of this, even though not specifically restricted to 60Co, to this day, the health effects on the personnel involved in these tests continues to be investigated.

Gerald England, licensed under CC BY-SA 2.0
The discovery of our element, cobalt, is an interesting tale, and one involving a fascinating man. Its credit goes to the Swedish chemist and mineralogist Georg Rushd Brandt, whose interest in metallurgy undoubtedly stemmed from his father. He was the eldest son of an extremely successful former apothecary (Georg senior’s pharmacy in Stortorget, Stockholm is still operational to this day).
In 1690, four years before Georg’s birth, his father (Georg Jürgen Brandt) bought land in Riddarhyttan, northwest of Stockholm to build a manor house. This was rich mineral land, lying in a small but well-established ore belt. The land included mines*, ironworks, roasting ovens, copper cabins and smelters (some of the oldest in Sweden), mills, and associated metallurgical industries, which Brandt Sr. took under his wing.
(*from which iron, and copper had been extracted and processed for centuries. Indeed, iron has been extracted from the ‘red earths’ of the Riddarhyttan area since around 700 BC).
These industries flourished under Brandt Sr.’s ownership, and as a mining entrepreneur he enthusiastically conducted chemical and metallurgical experiments, including his son in these from a very young age. His father taught him metallurgy, chemistry, and pharmacy, so minerals, ores, and chemicals became very important to the young Georg.
He was an exceptionally bright child. Indeed, in 1705, at the age of 11, the young Georg Brandt began studying (and, some say, teaching) at Uppsala University. He joined the Swedish Bureau of Mines in 1714, working for them for seven years before going to study medicine and chemistry at Leiden University (the epicentre of scientific research at that time). There he was taught by the famous Dutch doctor Herman Boerhaave, the founder of clinical teaching. Georg was greatly influenced by Boerhaave, who taught rationally, systematically, and scientifically, without the quasi-mystical and alchemical aspects of medicine and the ‘sciences’ so common at the time.
Georg went on to gain his doctorate in medicine at Université de Reims Champagne-Ardenne in 1726. Though he never practiced as a doctor, he was one of the physicians called to the deathbed of King Fredrik I, such was his good standing in the monarch’s eyes, for his “security and seriousness in dealings”.
Returning to Sweden in 1727, he resumed his association with the Bureau of Mines (completely revitalising their laboratories as their new director), before being appointed warden of the Stockholm mint in 1730. Later still he was awarded a professorship in chemistry at Uppsala University.
Much of his efforts entailed checking the purity of metals and analysis of minerals to determine their composition. He published his findings relating to gold and mercury in 1731, then in 1733 his research on arsenic (which had previously been understood as a kind of sulphur), its compounds and alloys was published. This was ‘De arsenico’, in Acta Literaria et Scientiarum Sueciae.
Georg’s work was outstanding, groundbreaking, in fact. He devised a completely new chemical classification, the ‘semi-metals’ (now known as metalloids), known primarily by their oxides or sulphides, in which he included arsenic, bismuth, antimony, and zinc.
During this time, he began to analyse minerals from his father’s land, including ores from the rich Pellugruvan copper mine. One such was a so-called ‘false ore’ which, based on some of its properties, resembled copper ore. However, upon smelting this did not yield metallic copper, but instead foul-smelling, poisonous fumes. It had long been known that these ores (known as ‘zaffer’ or ’smalt’) gave a beautiful blue colouration to glass. In 1735, Georg hypothesised that the intense blue colour, which had previously been ascribed to the presence of bismuth, was actually attributable to the presence of an unknown metal or semi-metal.
His experiments allowed him to separate this new element (cobalt) from bismuth. This not only proved that the blue colour derived from this new kid, not bismuth, but Georg became the first person since prehistory to identify a new metal (actually, he originally classified it as one of his ‘semi-metals’). He named this new substance ‘cobalt regulus’, and the first accurate description of metallic cobalt was published in his 1735 ‘De semimetallis’, in the Acta Literaria et Scientiarum Sueciae.
In 1742 Brandt isolated new samples of cobalt from sulphide ores (linnaeite, Co+2Co+32S4), again from his family’s lands. These cobaltous ores did not contain arsenic—a much safer proposition! With easier access to the new element, he was able to demonstrate that cobalt is ferromagnetic, and forms alloys easily with other metals such as iron, aluminium, copper, gold, and antimony.

SharpieType301, 2023, images from Chemical Elements, licensed under CC BY 3.0
But what of that name, ‘cobalt’? Well, that has a story too. Remember that Georg had used a ‘false ore’ to first isolate our element? These were well known to miners and metal producers and loathed as being ‘worthless’, robbing them of the valuable metals they had sought. The presence of these ores was thought to be pranking or punishment doled out my mythical malevolent underground creatures who inhabited the rocks and mines.
These were the ‘kobolds’, themselves believed to be expert miners and metalworkers. The word ‘kobold’ is medieval German, dating back to at least the mid-1300s, which means something akin to ‘goblin’ or ‘evil spirit’. There are parallels in Britain’s mines, with the Cornish ‘knockers’, Welsh ‘coblynau’ and ‘bluecaps’ from northern English mines. Given his upbringing, Georg was very well acquainted with this folklore, so this new element’s name was chosen as a hat-tip to the mythical creatures of his childhood… or maybe as a tribute to them. Who knows.
Before we get to its uses, which are widespread, let’s take a look at where cobalt comes from. It is quite a rare element, and it makes up only about 0.001% of the earth’s crust.
However, only rarely will you find it in nature in elemental form, as it reacts readily with oxygen (albeit usually at high temperatures), forming oxides that are fairly inert. So, on earth, it usually only occurs in compound form (bonded to atoms of different elements), including oxygen, but also sulphur and arsenic.
In nature these compounds are found as a range of ores (sulphide, arsenide and sulpharsenide ores), with some of the most important being:
- cobaltite [CoAsS]
- erythrite [Co3(AsO4)28H2O]
- glaucodot [(Co5Fe0.5)AsS]
- smaltite [CoAs2]
- skutterudite [CoAs3]
- carrollite [Cu(Co,Ni)2S4]

Robert M. Lavinsky, except smaltite Didier Descouens,
all licensed under CC BY-SA 3.0
Typically, these and its other ores (there are over one hundred and twenty-five different ores, thirty with cobalt in commercially valuable concentrations) are not found alone. Instead, time and again, they are associated with the ores of other metals, particularly copper (some 60% of cobalt comes from copper deposits) and nickel (accounting for some 38% of cobalt mined). This means that cobalt has long been, and still is, extracted as a by-product of extracting other metals.
In fact, only some 2% of the world’s cobalt is extracted as a metallic ore in its own right. This comes from extremely rare volcanogenic ore deposits in Morocco and from some arsenide ores in Canada. Volcanogenic ores come, as one might imagine, from volcanic rocks.
However, these deposits actually derive from ancient hydrothermal processes, where cobaltous minerals, principally sulphides, were laid down as precipitates from mineral-rich seawater solutions which passed through hydrothermal vents on the beds of ancient seas hundreds of millions of years ago. Remarkably, this process is ongoing—the modern equivalents are the fascinating deep-sea vents we call ‘black smokers’.
Interestingly, given cobalt’s affinity for oxygen, it is purported to have been identified as a naturally occurring native metal in both Russia, and in Canada in relatively recent times. It was first recorded as a free metal in 1994 by the Russian mineralogist Margarita Ivanovna Novgorodova at the Aidyrlya gold deposits, in the Orenburg Oblast (or region), in the southern Ural mountains. It has also been observed in Canada, specifically in Northeastern Ontario (at the Canadian Lorrain Mine, a.k.a. Maidens Mine). As a metal rather than a compound, it is also present in small quantities in the alloys that make up meteoric iron.
Although cobalt is mined in a fair number of countries, e.g., Australia, Azerbaijan, Canada, Cuba, Finland, Indonesia, Russia, the United States, and Zambia, currently the Democratic Republic of the Congo (known until 1997 as Zaire) is the largest producer. DRC supplies over 70% of the world’s cobalt ores. In fact, cobalt was first identified there in copper ores at the Katanga mine in 1914. Extraction began in 1924 and Congo rapidly became the world’s biggest producer, circumstances that have remained the case ever since.
These cobalt-rich primarily come from the southeastern region of DRC, Katanga, specifically from Lualaba and Haut-Katanga provinces at the southernmost tip of this vast country (there is only one country in Africa larger than the DRC, Algeria, and DRC is 11th in a list of the largest countries in the world).
DRC in not only huge (and a country with a tumultuous history, see our friend A W Kamau’s excellent series for more detail), but it is also an incredibly rich country, one of the world’s richest in natural resources. Cobalt ores are not the only treasure this region has to offer. In fact, copper, cadmium, diamonds (both industrial grade and gem-quality), gold, silver, zinc, manganese, tin, germanium, uranium, radium, bauxite, iron ore, and coal are all plentiful. Why then can it be the third poorest country in the world? More on this later.
But, for now, back to our element, cobalt. It is also quite unusual in that, for a given deposit of ore (that is, an accrual of minerals within a rock mass), the ore can include an assortment of differing cobaltous mineral types. This very unpredictability of cobalt ores, in chemical terms, makes developing a standard extraction process problematic as there are few, if any, that can accommodate the variation of different minerals in a single batch of ore.
In terms of refining our metal from its ores (we’ll come to how it’s refined later), China is the world’s leading producer, extracting nearly three-quarters of the world’s cobalt in 2022. Not too surprising, since China is also the world’s leading consumer. The Chinese obtain most of their cobalt from DRC, and more than 80% of this goes into the rechargeable battery industry.
Interestingly, because the interest in acquiring this metal is so high (we’ll get onto that too, later in this piece), some unusual avenues for obtaining it have been explored. A potential source for cobalt has been identified in the polymetallic deep-sea nodules and crusts on ocean floors. Remember those ‘black smokers’? These hydrothermal vents are primarily found along mid-ocean ridges. Hence, the mid-Pacific is a known source, but the Atlantic, and Indian oceans have also been flagged as ‘of interest’. There are thought to be over 120 million metric tons of cobalt available in these reserves… if this resource can be exploited.
Let’s take a look at some of the uses for cobalt and its compounds. Some of the earliest known use comes from ancient Egypt faience (a sintered-quartz glass-like ceramic material which pre-dates true glass). Objects created with faience were viewed as ‘magical’, having captured the sun’s shimmer. They were often associated with the idea of, fertility, the afterlife and rebirth.
We tend to think of faience as having a characteristic turquoise blue colouration, but this increasingly began to change during the second millennium BC. Now faience begins to be seen in other colours, including a deeper, richer blue, and a deep purply-black.
Although there is evidence for the production of faience back to around 5,500 BC (from a workshop near the temple of the jackal-headed deity Khenti-Amentiu, in Abydos), these colour changes coincide with the beginnings of glass production which took place during the Eighteenth Dynasty, around 1,500 BC. Although true glass is known to have been produced in Mesopotamia earlier than in Egypt, the earliest glass making factory yet to be excavated was discovered at pharaoh Akhenaten’s capital, Tell el-Amarna.
There was substantial trade in this desirable material, whether produced in Egypt or the regions to the east. Similar glassy, or glass-like materials, has been found around the Aegean area, dating to the same period as Amarna, and it was certainly in use right through to the 7th–6th century BC classical Roman city of Pompeii. So prized was it that the infamous Roman author and voluptuary Gaius Petronius recorded an anecdote about it in his 1st century ‘Satyricon’:
“…there was an artisan, once upon a time, who made a glass vial that couldn’t be broken. On that account he was admitted to Caesar [Emperor Tiberius] with his gift […] Caesar said to him, “Is there anyone else who knows how to make this malleable glass? Think now!” And when he denied that anyone else knew the secret, Caesar ordered his head chopped off, because if this should get out, we would think no more of gold than we would of dirt.”
The distinctive turquoise colour most commonly seen in faience (like the hippo, below) comes from copper compounds included the mix, usually copper(II) oxide, CoO, sintered with aluminium (III) oxide, Al2O3). The deep purple to black colouration seen in the wigs of some ushabtis (middle image below) reflects the use of manganese, and the deeper blues from cobalt compounds (Akhenaten seal ring, below).
Recent analysis suggests that the source of this element could have been cobalt alums, which are found at the western desert oases of Kharga and Dakhla in Egypt, due west of Luxor, a region where some of Egypt’s oldest rock formations are found, with evidence of ancient hydrothermal vents.

Hippo, Brooklyn Museum, Uhsabtis, Gary Todd, Akhenaten seal ring, Walters Art Museum all Public Domain
I mentioned that the use of cobalt compounds in faience arose with the beginning of glass production in Egypt, but glass as a substance actually originated much earlier (see A Glass Half Full), and with it, the use of cobalt.
What we know as glass has its origins as a coloured glaze from Mesopotamia (the Middle Eastern lands around the Tigris and Euphrates rivers) in about 3,500 BC, where glazes of a variety of colours were used for centuries. It is thought the blue glazes might have come into use a little later, but there isn’t clear evidence one way or the other as yet.
The earliest known object (to date) which has been analysed and proved to contain cobalt is a blue glass lump dated to about 2,000 BC from the site of Eridu, a Sumerian city in southern Mesopotamia (modern Iraq). This is thought to have been for use in a glaze, not as part of a glass object in its own right (although the first objects made exclusively of glass in Mesopotamia date to c. 2,500 BC).
The beauty of these coloured glazes can be seen in all kinds of vessels and statuary, and cover a considerable portion of Mesopotamian history, but I think the cobalt blues come to life best with the meticulously planned tiling which once adorned the massive Ishtar Gate from Babylon.
This edifice was constructed around 575 BC at the orders of King Nebuchadnezzar II. We were fortunate to see some small sections from this incredible structure for ourselves whilst visiting the Istanbul Archaeology Museum a few years back.

SharpieType301, 2006
The blues imitate the colours of highly prized precious stones, the deeper blue mimicking lapis lazuli, and the lighter hues possibly emulating turquoise. The bricks to which these glazes were applied were clay of a particularly fine texture, which had been pressed into wooden forms to create bricks of a regular size and shape. Glazes were painted on in the form of a finely ground frit (a form of glass) before the bricks were re-fired to produce a jewel-like, lustrous, vividly coloured surface.
Such decoration was obviously important to the Mesopotamians, and it was often found in association with royal and religious structures. Some of a series of forty-two ‘Sumerian Temple Hymns’ (recorded in cuneiform on clay tablets) thought to date to 2,300 BC, possibly back as far as 2,600 BC, have been translated. A number of these mention ‘lapis lazuli’ (quite likely in the form of cobalt glazes, rather than the real deal, which was excruciatingly expensive), for example:
“… The temple is constructed with gold and lapis lazuli, Its foundation on the nether-sea (apsu) is filled in. By the river of Sippar (Euphrates) it stands. O Apsu pure place of propriety, Esira, may thy king stand within thee. …”
This technology, the use of cobalt ores to produce rich blue hues spread to other areas of the world, probably through Muslim traders via the Silk Road routes.
The Chinese began colouring glass with cobalt to create a blue material during the Chou dynasty (from 1,122 to 221 BC) and it is thought that they may well have imported the cobalt they used from Persia. Although there are other sources of cobalt, it is believed that the Qamsar (also called Kāshān) mine in Persia was the primary source of cobalt ores in the ancient world, a position they maintained until at least the late Middle Ages, some say to the early 20th century.
Cobalt ores, in Persian sang-i lajvard, which translates to ‘blue stone’, were of great consequence to the Persians, as they provided a less costly imitation of the lapis lazuli which was transported from the Sar-e-Sang mines of Badakhshan, a region now in north-eastern Afghanistan. A great deal of Persian art and architecture benefitted from the contrast between the vivid blue and the gleam of gold.
Interestingly, the Chinese do not seem to have taken to using cobalt in ceramic glazes in the initial period of its availability, and the earliest known cobalt used on porcelains dates to the Tang dynasty (618–907 AD). It took rather longer for cobalt to find its way to European glazed ceramics. Tin-glazed pottery, decorated with a range of colours, including the blue cobalt was made in Iraq in the 9th century, gradually spreading east towards the Moorish regions of southern Spain the 13th century.
From here, it was traded and shipped to Italy by Majorcan traders, in the process becoming known as ‘majolica’. The Italians acquired the secrets of the blue glaze, and their own ‘maiolica’, particularly ‘berettino’ and ‘turchino’ wares from Faenza* near Ravenna, became well-known from the mid-15th century.
(*note the name Faenza became the French ‘faience’, the term now also used for the much earlier Egyptian frits. Confusing, eh?).
During the 1760s and 1770s, British potters got in on the act, not emulating the European ceramics though. They attempted to produce the distinctive blue-white glazes that looked like the popular, but extremely expensive, Chinese porcelain. To do this, they added cobalt oxide (CoO) to the lead-based glazes used for creamware (made with white clays from Dorset and Devon) that Staffordshire potters had developed around 1750, to give a blue-white gleam.
Perhaps the most famous producer of creamware was a man I have mentioned on several occasions in the past, Josiah Wedgwood, although it was another potter, the older Thomas Whieldon, who went into partnership with Wedgwood to accomplish this. Wedgwood’s highly desirable cobalt-assisted creamware went by the name of pearl ware, and this and other manufacturers’ products pretty much replaced the tin-glazed faience and majolica wares throughout Europe.
I can’t leave cobalt and its use in glass without a mention of a particular type of glassware which is very dear to my heart. This is Bristol blue glass, and as you’d imagine, this gets the glorious coloration it is renowned for from cobalt, in fact the same form of cobalt oxide as used by Wedgwood in his glazes.
Trade in this beautiful glass was of huge importance to the industrial success of the city (in 1720, Bristol was said to be the greatest and richest port in Great Britain). As it was so greatly valued as a status symbol and because it was priced accordingly, Bristol blue was available only to those wealthier customers.
It came about because a Devon chemist, William Cookworthy, had managed to make hard-paste porcelain for the first time in Britain, in 1768. This meant that now, a high quality ‘Plymouth porcelain’ was available, which was very like the material imported from China. Cookworthy joined forces with a Bristol potter and merchant, Richard Champion who (like Wedgwood) was trying to imitate the desirable ‘blue and white’ Chinese porcelains.
Being a smart operator (as well as a pharmacist, inventor, and an English Quaker minister), in 1753, Cookworthy bought the rights to all of the ‘smalt’ (cobalt oxide) produced in Saxony. This made cobalt rather more inexpensively available to Bristol craftsmen, and those of the Staffordshire potteries (indeed, a factory to smelt cobalt was established at Cobridge, just north of Hanley at the heart of the Potteries, about 1772). Thus, cobalt began to be incorporated into glassware and a legend was born.
Even glass and ceramics are not the only artistic avenues for our element though. In 1799, the French chemist Louis Jacques Thénard developed an inexpensive blue pigment (now known as Thénard’s blue or cobalt blue) for use in paintings. This was taken up enthusiastically by some of the great Impressionist painters (Claude Monet and Pierre-Auguste Renoir, amongst others), by the English Romantic painter J. M. W. Turner, and by the Dutch Post-Impressionist painter Vincent Van Gogh.
Cobalt blue is now a staple of the artist’s palette, and this gives me carte blanche (or do I mean carte bleu) to include one of the most iconic of paintings to use this rich, vibrant pigment. One which needs no real introduction: ‘Starry Night’.

Christopher Penn, licensed under CC BY 2.0
It isn’t only the pigment ‘cobalt blue’ which makes use of our element. Several other pigments, for oils, acrylics, and watercolours (even for car paints), are also cobalt based. Cerulean blue (in both of its forms), cobalt green, cobalt violet, cobalt purple, cobalt black, and cobalt yellow all make use of different cobaltous compounds, to produce beautiful colours in their own right, with a range of further differing shades created by mixing these with other pigments.
But what of other uses for cobalt and its compounds?
Cobalt carboxylate compounds (also known as cobalt soaps) are used as siccatives, drying agents for inks, paints, and varnishes. They work by oxidising the oils in these liquid coatings and are a favoured choice, in fact the most commonly used, as they produce little discolouration in the coating as applied, nor when these dry.
These same carboxylate compounds may also be used as catalysts (increasing the rate of a chemical reaction). Likewise cobalt(II) acetate, Co(CH3CO2)2·4 H2O, is used as a catalyst in the production of ‘PET’ plastics, the polyethylene terephthalate (a.k.a. polyester) from which many plastic bottles are made, and clothing fabrics are produced.
Other cobalt compounds, such as dicobalt octacarbonyl, Co2(CO)8, are also used as industrial catalysts, for example, as part of the Fischer–Tropsch process to produce liquid hydrocarbons (to be used as a fuel) from carbon monoxide and hydrogen, known as ‘syngas’.
Furthermore, cobalt-molybdenum catalysts are the favoured catalysts for the desulphurisation (removal of sulphur and sulphur-containing compounds) from natural gas and refined petroleum products (e.g., petrol, jet fuel, diesel, and fuel oils). This process, known as hydrotreating, can also remove other impurities from crude oil, including nitrogen-containing compounds, such as nitrous oxides, pyridines, and amines.
When we looked at our last element, titanium, its use in medical devices and implants was highlighted. For very similar reasons, e.g., corrosion resistance, mechanical properties, and biocompatibility, cobalt and its alloys have recently made advances in the biomedical industry. For example, ‘vitallium’ alloy, which is 65% cobalt, 30% chromium, 5% molybdenum is used in artificial joints, and in dentistry. In addition, cobalt ferrite (CoFe2O4), used in nanomedicine (an increasing area of research), has been demonstrated to exhibit good antimicrobial activity against all tested bacteria.
One difference is that titanium plays no part whatsoever in human metabolism, whereas cobalt has a very significant role in the human body. Do you remember the table we’ve seen in previous articles, showing elements which are crucial to our existence? Cobalt is there too, as a trace element highlighted in green.
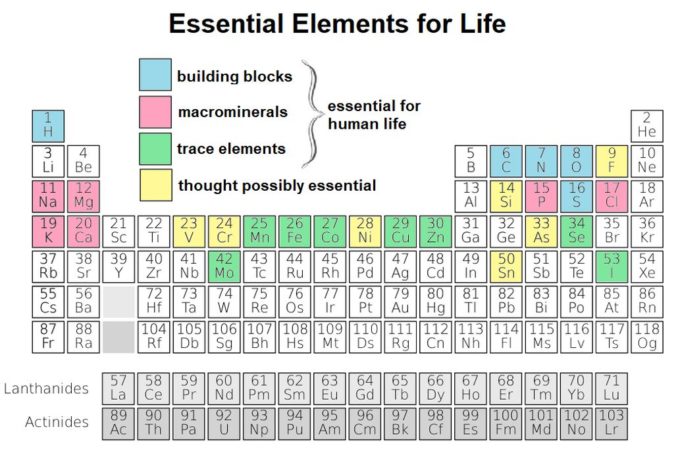
SharpieType301, 2023, adapted from Cepheus, public domain
In fact, our element is necessary to the metabolism of all animals, not only humans. Not least, it drives mechanisms to ensure blood and nerve cells remain healthy, and red blood cells are produced in the bone marrow in sufficient quantities. Cobalt also contributes to the production of DNA.
Cobalt is a key element in cobalamin, also known as vitamin B12 (which has the chemical formula C63H88CoN14O14P), one of eight B vitamins found in meat, eggs, and dairy products. Incidentally, vitamin B12 is the most chemically complex vitamin the body requires.
We humans, at least, those of us who eschew an entirely vegan lifestyle, usually readily assimilate cobalamin from the meat, fish, eggs, and dairy foodstuffs included in our diet, since animals store vitamin B12 in liver and muscle tissues, amongst other places.
That said, the most common cause of vitamin B12 deficiency in the UK, is an autoimmune condition called pernicious anaemia, whereby the body is unable to absorb vitamin B12 from the diet, even if foods rich in this vitamin are consumed. Common symptoms can include severe fatigue, weight loss, headaches, and possibly muscle weakness, numbness or tingling in hands and feet, as nerves are not functioning as well as they should. Despite not being resolvable, thankfully, periodic injections of vitamin B12 can help treat this condition.
However, ruminant livestock (e.g., sheep or cattle) are not meat eaters, so rely on trace elements in the soils in which the plants they eat grows. In their stomachs, useful bacteria convert the cobalt taken up by plants into vitamin B12. That said, lack of this element in the soil can lead to a wasting disease, so-called ‘bush sickness’, that makes the animals look as if they were starving, even though they are getting sufficient to eat. This was a problem for New Zealand farmers in the past, particularly on the North Island where the volcanic pumice soils are deficient in cobalt. Thankfully, adding cobalt supplements to the animal’s feed sorts this out.
That said, too much cobalt in our diet can be as harmful as a deficiency. In the mid-1960s this became apparent in Quebec, where cobalt sulphate had been added to beer by a renowned brewery to stabilise the foam.
Unfortunately, indulging in their favourite, but now cobalt-laden, tipple (Dow Ale) caused more than twenty people to lose their lives, as they developed ‘beer drinker’s cardiomyopathy’ before anyone figured out the cause!

vieillespubs, Public Domain
The brewery quite sensibly dumped the remainder of its inventory of Dow Ale, which had been until that point the number one selling beer in Quebec, into the Saint Lawrence River.
Time to look at more beneficial uses. One of metallic cobalt’s most important applications is in the production of ‘superalloys’, accounting for somewhere in the region of 20% all cobalt produced. These are alloys specially formulated to provide excellent mechanical strength, with a stable surface, resistance to both corrosion and oxidation, and the ability to withstand deformation caused by thermal creep.
As such, superalloys are used where metals are exposed to great stress and high temperatures, for example in gun barrels, and turbine blades in jet engines and gas turbines. These high-performance alloys are, in the main, composed of iron, cobalt and nickel. However, small quantities of other metals, including chromium, tungsten, aluminium, and titanium, may be present.
Loosely related to this, cobalt metal is, from time to time, used in electroplating. This improves the objects to be plated, as cobalt has an attractive appearance, and its hardness and resistance to corrosion bring additional advantages.
Cobalt’s magnetic properties are of use in alloys as well. Such alloys are used in electric motors and generators, and recording devices, making up in the region of 7% of the demand for cobalt metal. They are crucial where a metal is required that will maintain its magnetic field. You may well have heard of Alnico strong, permanent magnets. Well, the ‘co’ in the name stands for cobalt. The ‘al’ is aluminium, and the ‘ni’ is nickel, although the alloy used to make the magnets includes iron, and may also include small quantities of copper, and occasionally titanium.
Cobalt is one of the metals used for the production of cemented carbides (sometimes referred to as ‘cermets’), a class of extremely hard, tough, durable materials used widely for cutting tools. Their advantages include leaving a good surface finish and that such tools can be used at significantly higher speeds than standard steel equivalents.
Cemented carbide comprises fine particles of a metal carbide (chemical compounds of metals such as tungsten, titanium, or tantalum and carbon) and a ‘binder’ metal, in this case cobalt. This type of cutting tool is often used in mining and tunnelling but are applicable to a wide range of other applications. Use in this form accounts for some 12% of the metallic cobalt produced. Interestingly, one of the cemented carbides, tungsten carbide, although somewhat brittle, has become a popular choice for jewellery.
But all these uses pale in comparison to the 58% or so of cobalt consumption worldwide used in the production of rechargeable batteries. The batteries are largely those powering hybrid and electric vehicles (EVs), but cobalt is actually in every lithium-ion rechargeable battery made. This includes the batteries that power our smartphones, tablets, laptops, smart watches, electric toothbrushes, etc.
This level of use is, of course, increasing by leaps and bounds as the world goes crazy to move towards zero-emissions.
On that note, it’s probably time to ask that somewhat sizeable pachyderm, having waited patiently in the room until this moment, to take a bow.
In 2019, The Right Honourable Chris Skidmore signed legislation on our behalf which legally bound our country to committing to end our contribution to global warming by 2050. For more detail, see the United Nation’s 2015 Paris Agreement.
In doing so, the UK not only became the world’s first major economy to make such a pledge, but our 2050 net zero target is one of the most ambitious in the world. You were, of course, asked whether you agreed with this beforehand, right? Cheers, Chris.
OK, so what actually is this ‘net zero’ stuff? Well, McKinsey & Company define this as:
“an ideal state where the amount of greenhouse gases (GHGs) released into the earth’s atmosphere is balanced by the amount of GHGs removed.”
They go on to add that in order to reach net zero (it is impossible to do so by cuts in emissions alone), decarbonisation will be needed. In practice, we are told, this can be accomplished by moving to energy sources or materials that emit less carbon.
Carbon, eh. What they mean by this is a natural, and in fact essential, component of the Earth’s atmosphere, carbon dioxide gas, CO2. This gas has been named Enemy No. 1, and all this has been deemed essential, as the University of Oxford proclaim that:
“Global warming is proportional to cumulative CO2 emissions, which means that the planet will keep heating for as long as global emissions remain more than zero.”
This ‘global warming’, our planet ‘heating up’ uncontrollably and permanently will, we are reliably informed, lead to catastrophic consequences.
OK, but where does our element, cobalt, fit into all this? With ‘net zero’ and ‘climate change’, one of the primary targets is the combustion of those naughty fossil fuels, which we are assured produces some 83% of global CO2 emissions. I can’t help but think a few volcanoes might take issue with this statement and suggest that we could ‘hold their beer’, but what do I know, eh.
However, electrification is the name of the game, that is replacing as much as possible that currently uses fossil fuels, with their electrically powered equivalents. This means things like internal combustion engine vehicles with electric vehicles (EVs), and gas boilers with energy sources such as heat pumps, wind, and solar power, the last two needing battery storage facilities. It is this arena that cobalt takes centre stage.
We have already seen that non-fossil fuel vehicles, whether they be hybrid or electric, require cobalt. Two years ago, in 2021, markets saw the demand for cobalt for electric vehicles (EVs) overtake all other battery applications, including those required to store energy from solar and wind farms. Taking a huge 34% of all cobalt produced, this one sector became the largest end user. EVs are expected to consume around half of the cobalt produced, worldwide, by 2026.

benjie castillo, licensed under CC BY 2.0
On average, a typical EV car requires somewhere in the region of 13.3 kg of cobalt (the battery pack alone grabs c. 10 kg of that). At the end of 2021, the number of electric cars on the roads, across the globe, stood at c. 16.5 million. So, these vehicles alone, before you consider other batteries, larger commercial electric vehicles, and other applications, required something like 219,450 metric tons of our element (1 ton = 1,000 kg)—that’s a lot of cobalt.
We have also seen that, in a given deposit of cobalt-bearing ore a variety of differing mineral types can be found, making a standardised extraction process rather difficult to achieve. For the techniques by which cobalt can be extracted from different ore types, see Wiki’s Cobalt extraction page. This leads into the fact that, for each ton of cobalt extracted, on average, approximately 1,500 tons of ore must be mined and processed.
So, let’s have a little look at how it is mined, and by whom.
In 2021, 170,000 tons of cobalt were produced, globally. Of that, 70% (c. 120,000 tons) came from the Democratic Republic of the Congo (this figure rose to 130,000 tons in 2022 and is still rising).
There are five major cobalt producers operating in DRC. Swiss-based Glencore is the largest of the ‘big boys’, having produced an estimated 25,320 metric tons (MT) in 2021. The privately held Eurasian Resources Group is the next largest, with 20,700 MT, then China Molybdenum (part owned by the Chinese government) accounting for 14,800 MT. Gécamines (the DRC state-controlled company) follows up with 13,860 MT, and then there’s Zhejiang Huayou Cobalt, accounting for 5,390 MT.
So that gives us around 80,070 tons. Where does the rest of that 120,000 tons come from?
There are, of course some smaller players too, but around one fifth (some 24,000 MT) of all the cobalt produced in DRC is generated by so-called ‘artisanal’ miners (gosh, doesn’t that sound a nice wholesome cottage industry, ‘artisanal’).
In truth, that mass of cobalt ores is dug out of the ground, by hand, in the most appalling of conditions. The miners dig in pits, often tens of metres in depth, tunnels, trenches, and spoil heaps. These are often quite unsupported, so collapses and cave-ins are commonplace, and are unventilated, so the risk of suffocation is not insubstantial. The miners use chunks of rebar, chisels, shovels, or any simple tool that comes to hand.
The rates of injuries and deaths is colossal, since this practice is unregulated and pretty much none of the miners work with even the most basic of protective equipment. These people also face chronic exposure to cobalt-laden dust risking a potentially fatal lung disease (hard metal lung disease). Sustained skin contact with our element causes horrific dermatitis, which cannot resolve as repeated contact with the cause occurs.
It’s been calculated that there are about 150,000-200,000 artisanal miners. Worse still, a good number of them, up to a third by some estimations, are children (some as young as four years old work on the surface, sorting or washing the ores). Similarly, mothers even work with their babies strapped to their backs.
Australian broadcasters ABC News produced a video just last year, which gives an overview of the cobalt mining in DRC: ‘Blood Cobalt: The Congo’s Dangerous and Deadly Green Energy Mines’.
Once again, in 2021, the state-controlled Gécamines, created a new subsidiary called Entreprise Générale du Cobalt, a state initiative to ‘formalise’ and take control of the country’s artisanal cobalt-mining sector. It was hoped that this would improve conditions for the thousands of people involved in artisanal mining. A few years on, how has this worked out? Well, they have a fancy website, but I’ll give you three guesses. It is at this point that I will remind readers that DRC is the third poorest country in the world.
Even for those employed by the big boys, working conditions are better, but still far from ideal. It’s said that the miners have what has been described as ‘a slave and master’ relationship with their employers. Employees may at least be supplied with ‘some’ personal protective equipment, but they work extremely long hours, sometimes in unsafe working conditions, with limited access to water (in intense heat) or food, and for such little pay that basic living expenses are barely, if at all, covered.
Cuts in pay for various ‘infringements’, for example taking time off through sickness, are not uncommon. The miners cannot argue—if they do, they are simply fired. Glencore seems to have the best record, but even this is pretty dire. Perhaps unsurprisingly, the Chinese organisations are way down the list (physical abuse is not uncommon, reportedly). All this said, things are even worse for the many workers taken on through subcontractors.
But it isn’t only the miners themselves who are affected by the atrocious conditions surrounding the production of cobalt. Environmental pollution is colossal, and whole communities have been uprooted and displaced. This often happens with minimal consultation but with vague promises of a better life, because a new mine is to be developed or an existing one expanded. A picture of the effects on those people who are not miners, but still losers in this global game can be seen in the SOMO articles ‘Cobalt blues’ and ‘Katanga Calling’, as well as in a short documentary, ‘Whose Wealth? Cobalt from Congo’.

Adapted from Paul Stevenson, licensed under CC BY 2.0
I could go on, but the point of explaining all this has been to highlight just one impact of that ‘Net Zero Strategy: Build Back Greener’ policy that Chris Skidmore kicked off on our behalf. I mentioned that ‘elephant in the room’, that green one, and there is much more, unrelated to cobalt, that might be said (not least about ‘green’ power systems), but those are not the purpose of this article.
I don’t wish to leave us with the cobalt blues, so I’d like to finish with just a couple of slightly cheerier things.
Did you know that cobalt has actually been mined commercially here in Britain? No, I didn’t either, but in fact it has. It has been mined from deposits in Cornwall, Cumbria, Cheshire, north Shropshire, and the Ochil Hills in Scotland. The minerals were primarily used for the manufacture of ’smalt’ as a colourant for ceramics, and glass.
Why? Well, whilst the main sources for this valuable material were, at that time, in Saxony, the Napoleonic Wars provided the impetus to look closer to home for indigenous supplies.
Cornwall was the leading UK source, with the Wheal Sparnon mine at Redruth finding a particularly ‘large and valuable cobalt lode’ in 1814. This then became known as ‘Wheal Sparnon and Corner Stone Cobalt Mines’ and was in the only mine Cornwall worked exclusively for cobalt ore. In 1814, two tons of cobalt ore were sold in London at a value of £1,200. The ores found at Wheal Sparnon included skutterudite, safflorite, and the beautiful pink purple to deep rose pink erythrite. By 1819, the ‘cobalt lode’ was worked down to some 60 fathoms, eventually reaching 70 fathoms (about 420 feet).
The Staffordshire Potteries were the main beneficiaries of the Cornish ‘smalt’, with mention of the British cobalt proudly displayed on the reverse of some of Spode’s ‘India’ pattern ceramics from 1816. In fact, some of the Cornish ores were almost certainly refined in Hanley, at the long defunct ‘British Cobalt Smelting Company’.
The colour this source of cobalt produced was so well respected, that a group of Staffordshire potters (John Yates, George and Charles Mason, and Josiah Wedgwood II, son of the original Josiah Wedgwood) signed a testimonial in 1817 to “the superiority of this pigment”.
As we saw, though, Cornwall was not the only cobalt source in Britain, and just last year an extraordinary find was made at Alderley Edge in Cheshire by the Derbyshire Caving Club. This was a cobalt mine, abandoned in the early 19th century, likely around 1810.
Amazingly, an intact windlass, and a variety of personal items (including clay pipes, a metal button from a jacket, and leather shoes) were found, still in place, as well as inscriptions written in candle soot. There were even fingerprints left in the clay used to hold candles, and a clay bowl, buried in a wall, which might point to an offering made by superstitious miners in gratitude for a decent vein. An incredible archaeological find, but not an easy one to see in person.
On a final note, in Timiskaming District, Northeastern Ontario, there’s a small town called Cobalt. Quite an odd name, you might think, for a town best known as ‘The Silver Capital of Canada’. Well, indeed, except that along with that valuable silver, another element can be found there. Yes, it was cobalt. By 1908 the region was not only the world’s largest producer of silver, but it was also the largest producer of the cobalt (a by-product of the extraction process and seen primarily as a bit of a nuisance) which gave the town its name. This ‘silver-rush’ lasted until the 1930’s when getting to the ore became more problematic as surface deposits were mined out, and the price of silver took a downturn.
In the last few years, interest in the geology of this area has picked up again, as cobalt as an element becomes more valuable. To be fair, it doesn’t harm that both silver and gold can be found in the rocks, and probably the spoil heaps too, and there’s even the possibility of diamonds!
To me though, I like the fact that the town was held in such affection at one point, that a song was written about it.
© SharpieType301 2023



