In previous articles, we’ve explored the development of the Periodic Table (a.k.a. the Periodic Table of the Elements), and examined the elements Hydrogen, and Carbon.
In this, the fourth, I’m going to take a closer look at an element which has long been one of my particular favourites, Mercury.
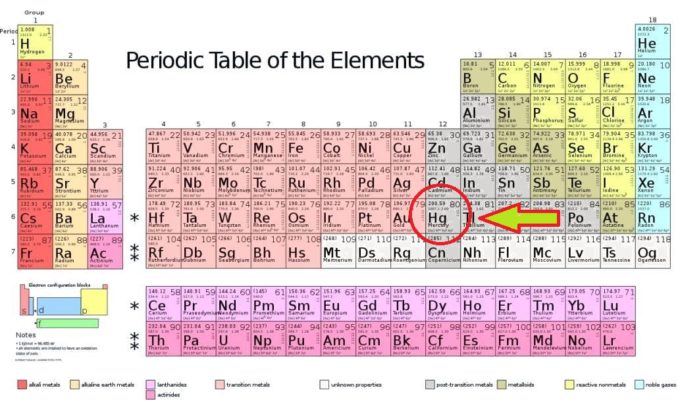
Modified from 2012rc, licensed under CC BY 3.0
As you’ll see from the Periodic Table above, mercury lies in Group 12. All of the elements in this group are metals, and compared to most metals are fairly soft. They include zinc (Zn), cadmium (Cd), and the synthetic radioactive element copernicium (Cn).
The Group 12 elements are all divalent (sometimes called bivalent), which means that each atom has two electrons available with which to form chemical bonds. Mercury, being a metal, forms ionic bonds, which we briefly touched upon in a previous article. For the true chemiphobes amongst you, skipping the next few paragraphs might be wise.
Ionic bonds are formed when a metal reacts with a non-metal. This is because the two elements exhibit a different degree of electronegativity. Electronegativity is the propensity of an element’s atoms to attract electrons, that is, to draw electrons towards itself in a chemical bond. This is influenced by its atomic number (i.e. how many positively charged protons are in its nucleus) and the distance from the nucleus its outer (valence) electrons are.
Electrons are arranged in orbits, a consecutive series of electron shells around the nucleus. This is a bit like the layers of an onion. In truth, it’s slightly more complex than this as the shells have subdivisions (sub-shells and atomic orbitals), but this simple analogy will suffice.
The key point here is that each electron shell can only hold a maximum number of electrons, and this is constant across all elements. There’s a useful list of elements, showing the numbers of electrons per shell, illustrating how the shells fill up, HERE.
Mercury is a transition metal, one of the numerous elements shown in salmon pink in the Periodic Table above. These are elements which only have a partially filled d orbital (electron sub-shell).
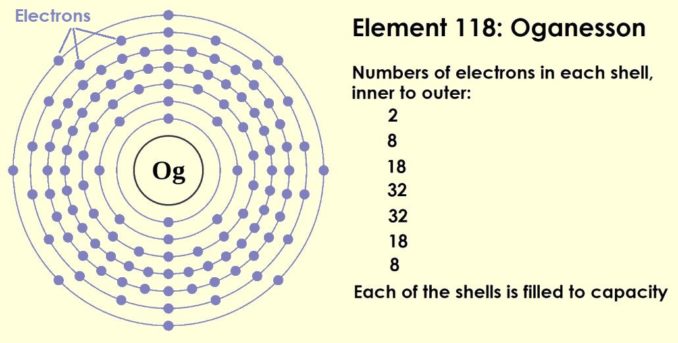
An atom is only truly stable (unreactive) when its outermost shell is filled to capacity. This is the reason that the inert or noble gases (helium, neon, argon, etc.) are such chemically unreactive elements. They have neither electrons to spare for use in reactions, nor are they missing any.
Oganesson, seen below has the highest atomic number (it’s the heaviest) of all the known elements. It’s a synthetic element in the noble group, and each of its shells, including the outermost, is filled to capacity.
Adapted from Pumbaa, licensed under CC BY-SA 2.0
Having explored the concept of shells and electrons, let’s have a look at ionic bonding in action.
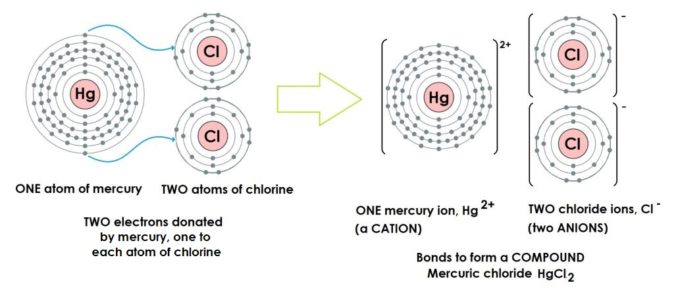
In the example below, the two chlorine atoms need to acquire just a single electron each to complete their outer shells. Mercury, conveniently, has two electrons in its incomplete outer shell that are available to be donated.
SharpieType301, 2023
Mercury has the following numbers of electrons in its electron shells: 2, 8, 18, 32, 18, 2
- The outermost shell is incomplete – only two electrons out of a maximum of eight
- Therefore, it is not stable so will react
- When these electrons are ‘donated’ to another atom (or atoms) in an ionic bond, this will stabilise mercury as the next shell back towards the nucleus is complete
Chlorine has the following numbers of electrons in its electron shells: 2, 8, 7
- The outermost shell is incomplete – only seven electrons, out of a maximum of eight
- Therefore, it is not stable so will react
- When an electron is ‘acquired’ from another atom in an ionic bond, this will stabilise chlorine as the outer shell is now complete
The compound produced by the example of ionic bonding above is mercury (II) chloride, HgCL2 (a.k.a. mercuric chloride, or corrosive sublimate). It’s a rather nasty piece of work, highly toxic and very corrosive. More of this later.
Anyway, enough of the heavy chemistry for now, let’s take a look at what is interesting about this beautiful element.
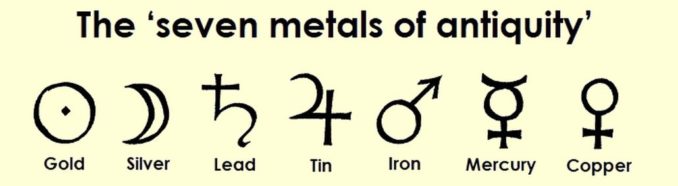
Mercury is one of the ‘seven metals of antiquity’, that is to say, the metals that were both recognised by our ancestors and used in ancient times.
SharpieType301, 2023
It almost goes without saying that, though known for millennia, it was French scientist Antoine-Laurent de Lavoisier (who we’ve met in previous articles) who first recognised and designated mercury as a chemical element. Prior to that, it was regarded as a property of all metals, a ‘principle’ or ‘essence’ at their core, which made them a ‘metal’.
Elemental mercury is a shiny, mirror-like liquid at room temperature, unique amongst metals. Being a liquid, it evaporates (off-gassing or vaporising) relatively easily. It is possible to solidify mercury, but only if you were to freeze it by subjecting it to chilly, but not extreme temperatures at or below -38.83ºC. As a solid, it likely crystallises under the rhombohedral system.
Even as a liquid, it is very dense. Nearly anything will float on mercury, and it is apparently feasible to use the surface of a mercury bath to play billiards, the phenolic resin balls only sinking by a few millimetres. Indeed, there is a famous photograph (which I cannot reproduce here) from the October 1972 edition of National Geographic, of a rather cheery looking gentleman sitting upright on a pool of mercury. Due to its high cohesive forces, he’s not even getting wet!
Mercury has been given the symbol Hg, which might seem counterintuitive until you realise that it was once called ‘hydrargyrum’, combining the Greek words for water (ύδωρ) and silver (ἄργυρος). The oldest written reference comes from Aristotle (c.4th century BC), who referred to it as ‘water silver’, ‘fluid silver’ or ‘quicksilver’. It has long been referred to as quicksilver, by alchemists amongst others, and it’s the only element whose alchemical name is still used, almost as often as its modern name.
The name ‘mercury’ derives from the Latin verb ‘to trade’ (mercari) and is linked to the planet of the same name. This small planet is the most rapidly moving in our solar system, and its name comes from the Roman god Mercury, he of the winged sandals. As the god of merchants, travellers, and trade, speed and agility were his traits. The metal mercury, quicksilver, was perceived to exhibit similar attributes.

GrrlScientist, licensed under CC BY 3.0
Mercury has an atomic number of 80: each mercury atom has eighty protons in its nucleus. Interestingly, in its natural form, mercury is actually a mixture of seven stable isotopes. These are: 196Hg (0.15%), 198Hg (9.97%), 199Hg (16.87%), 200Hg (23.10%), 201Hg (13.18%), 202Hg (the most abundant, at 29.86%), and 204Hg (6.87%).
It readily mixes with other metals to form alloys called amalgams. Alloys are slightly unusual; they are not usually chemical compounds (where the elements in their make-up are chemically bonded). Typically, they are simply mixtures, with atoms of each element intermingled rather than tightly bonded. The second metal could almost be described as ‘floating’ in the mercury, a little like crushed ice floating in water in a Slushie.
It is a diamagnetic element, meaning that it is repelled by a magnetic field—quite the opposite of what we normally see in daily life with metals like iron. Surprisingly, unlike most metals, it is also a relatively poor conductor of heat.
Mercury is a relatively rare element on Earth, accounting for only around 0.08 parts per million (ppm) of the Earth’s crust. It isn’t usually found in its native (liquid) state but, like many metals, more commonly as an ore. In this case, the primary ore is cinnabar (mercuric sulphide, HgS). Occasionally, depending on the conditions, pieces of ore can be found where, as well the characteristic red cinnabar, glistening globules of liquid mercury are visible.

Rob Lavinsky, licensed under CC BY-SA 3.0
Mercury’s main ore, cinnabar, is an interesting substance in its own right, having long been used as a red pigment. It’s the glorious ‘vermillion’, a firm favourite of Renaissance painters such as Titian, but there is evidence for its use dating back a great deal further.
The earliest use, yet known, was around 7,000-8,000 BC, from wall paintings at the Neolithic site of Çatalhöyük in Turkey. At Casa Montero, at a 5,300-5,200 BC Neolithic flint mine in central Spain, a ‘tool bag’ was found to contain a cinnabar coated flint blade. Its use is also known from Chalcolithic ‘beehive’ or ‘domed’ tomb burials in Spain, and in the Balkans, where it has been identified as decoration on ceramic figurines and vessels from Pločnik and other Serbian sites. Lengthy use indeed, but here’s a helpful tip. When using vermillion, don’t lick the brush to wet it. Your pretty paint is, like all of the mercury compounds, highly toxic!
Those of you who grew up around the same time as me will have encountered mercury in school science labs. If you were privileged and trusted enough to have assisted teacher in class (nope, that wasn’t me), you might remember that a tiny bottle of it was surprisingly heavy for its size.

Da Sal, licensed under CC BY 2.0
You will almost certainly have seen a glass thermometer filled with this bright, silvery substance at home too. The one mum stuffed under your tongue when you felt a bit off colour, with a strict command not to bite it.
You may have come across mercury in several compound forms at home too. Cuts, scrapes and abrasions were often painted with a viciously stinging paste or liquid by mums, aunties, and grans, quite often accompanied by a clip round the earhole for being so stupid… or was that just me? One of these, which turned your already sore and damaged skin a fetching yellow, was mercurochrome, an organic sodium salt of mercury. I won’t give its chemical name as it’s way too long, but the formula is: C20H8Br2HgNa2O6. It was, like its colourful companions iodine and gentian violet, a very effective antibacterial and antiseptic. Unfortunately, it was also, er… a bit on the toxic side.
Despite its toxicity, mercury and its compounds has a very long and varied history of medicinal uses, across all ages and strata of society. Dating back to c.500 BC it was viewed as an aphrodisiac in India and China. In traditional Chinese medicine, combining cinnabar with raspberry juice was thought to increase fertility in older men. Conversely, Chinese women are also said to have used mercury as a contraceptive. Well, I guess that’d probably do it.
Before becoming president, Abraham Lincoln regularly used medicine called ‘blue mass’ to treat his melancholia (we’d now call this depression). Unfortunately, the little blue pills contained not only liquorice root, rosewater, and honey, but pure liquid mercury!

Gage Skidmore, licensed under CC BY-SA 2.0
Thankfully, being a smart chap, he recognised that this remedy “made him cross”. Indeed, he experienced such ferocious rages that he trembled, and these furious outbursts made him shake fellow politician Orlando Ficklin until the poor man’s teeth rattled. There’s a good reason such a temper is known as mercurial!
Having ceased taking ‘blue mass’, and thus stopped poisoning himself, his mood changed dramatically. He became the calm, unflappable man who is remembered for his self-control when provoked. This continued throughout his presidency, right up until his assassination in 1865.
Another medical blast from the past, which some might remember by name, if nothing more, is calomel (Hg2Cl2). Selected for a variety of ills, calomel was seen as a bit of a miracle drug. Amongst other applications it was, not to put too fine a point on it, used as an exceptionally effective laxative. Indeed, the 8,000-mile route taken by American explorers Meriwether Lewis and William Clark as they travelled across the wilderness has been tracked by their use of the aptly named ‘thunderbolts’ or ‘thunderclaps’, the calomel pills they took to keep themselves regular along the way. This left detectable deposits of mercury in the latrine pits they dug near their campsites.
But it wasn’t just known as a laxative. As recently as the 1920s, parents would rub calomel-based powders into their baby’s gums to ease teething pain. Sadly, rather than helping the poor darlings, this led to some children suffering a ghastly condition known as Pink’s Disease, later (by the 1950s) recognised as mercury poisoning.
Oh, and the compound we looked at earlier to demonstrate ionic bonding, mercuric chloride (mercury (II) chloride, HgCL2), that unpleasantly corrosive one? This too was sold as an over-the-counter disinfectant. Furthermore, it was also recommended as the ‘go to’ treatment for the ‘great pox’ (syphilis) from the 16th century onwards and was still in use until as recently as 1916!
Unlike calomel, this compound is extremely water-soluble, which makes it readily absorbed by the body. Mercury is lipophilic (it is prone to dissolving in the body’s fats, oils, and lipids). This means that, once absorbed, mercury accumulates in tissues and organs like the brain and kidneys.
Generating intense controversy, it has been proposed that mercury poisoning, from the treatment of syphilis, might have contributed to the death, at just 35 years of age, of Wolfgang Amadeus Mozart. He certainly exhibited many symptoms of the disease in his final years. If this is true, he’d have been in pretty good company, as not only was syphilis commonplace, but a number of well-loved composers and musicians are known to have suffered with it.
In treating the disease, mercuric chloride was applied topically in the form of a salve and being corrosive, burned the parts to which it was applied. Believe it or not, this was seen as being a positive—if it hurt, it must be effective. If I’m honest, I don’t really wish to dwell too much on that, but the progression of this disease is so unpleasant that people went along with it, desperate for a cure.
Early onset treatments of this nature may have halted the progression of syphilis (mercury is, after all, antibacterial), but once it was well-established in a patient the disease was much harder to eliminate. This required increased dosages and adapting the types of treatment. In fact, as if this ‘salve’ wasn’t bad enough, steam baths were encouraged too. These, needless to say, had a little added medicinal extra, in the form liquid mercury. This of course evaporated in the heat so that quantities of toxic mercury vapour was inhaled along with the steam.
Because it acts as a neurotoxin, inhaling high concentrations of mercury affects the central nervous system (CNS). This in turn controls things like cognitive function, mood and personality, physical movement, breathing, heart rate, and body temperature. It is perhaps no great surprise that the treatment ended up being more unpleasant than the disease and, ultimately, lethal.
A pithy expression which relates to the use of mercury to treat this horrible disease is well-documented: “A Night with Venus, a Lifetime with Mercury”. The length of that lifetime, however, may not have been quite as expected.
Before I leave this rather unsavoury facet of mercury’s story, I’ll just point out that those of you of a certain maturity might have a few ‘silver’ fillings in your molars from childhood trips to the dreaded dentist. If so, were you aware that silvery dental amalgam contains mercury? Oh yes, and that the amount of mercury released from the amalgam increases as those fillings age? Ah but it’s alright, as rNHS says they are perfectly safe, so stop grinding your gnashers with worry!
Goodness, with all this it’s really rather a miracle we’re all still around, eh? But then, in the words of another ‘Mercury’, Freddie this time, Who Wants To Live Forever?
Although we’ve already seen that, as the compound cinnabar, mercury was in use from ancient times, so indeed was elemental, or liquid mercury. It appears to have been known at least back to the ancient Egyptians. In 1886-7, archaeologist Heinrich Schliemann (best known for his quest to find and excavate Troy) excavated small vials thought to contain mercury from Qurna which dated back to c.1,600-1,700 BC. It’s quite possible that it was known considerably earlier.
The Phoenicians and Carthaginians are reported to have traded mercury from the mines at Almadén, in Spain, by around 750 BC. This was used to extract gold and other precious metals by amalgamation (more of this technique later). Still exploited to this day, the mines are the largest mercury producing site in the world, said to have yielded more than 275,000 tons of mercury.
That liquid mercury was utilised in China by the second century BC is indisputable. Proof comes from the Mausoleum of Qin Shi Huang, (the First Qin Dynasty Emperor, he of the Terracotta Army). This was meticulously described by Sima Qian, an early Han dynasty Chinese historian, in his Shiji. The tomb complex, which is vast, was carefully laid out and constructed, and was designed to guarantee the Emperor’s immortality.
To emulate the Emperor’s kingdom in the afterlife, the tomb is said to have had seas and a hundred simulated rivers created using mercury, not merely static but flowing. This detailed underground world was set beneath a ceiling simulating a night sky, studded with pearls and precious gemstones to represent the stars. Interestingly, extremely high levels of mercury have been detected in soil samples from the area of the (as yet unexcavated) tomb, potentially tying in well with Sima Qian’s descriptions.

Wikimedia Commons, public domain
In those times, and for a very long period afterwards as we’ve already seen, mercury and its compounds were commonly used in many cultures as components of medicines and elixirs. Given its toxicity, that wasn’t really a great plan, and it’s a little ironic that Emperor Qin Shi Huang allegedly died (aged just 49) after ingesting a mercury-based ‘elixir of life’, one which was concocted for him by his alchemists and physicians to make him immortal.
However, the use of liquid mercury in China actually extends back to the 4th century BC, and the Zhou dynasty, possibly considerably further back still. Archaeometallurgist Dr Kilian Anheuser, a former colleague of mine, made clear that the principal technique for the gilding of metalwork at that time was fire gilding.
This was seen as an almost magical process, where a thin layer of gold (or, in fact, silver) could be applied to the surface of another metal. Fire (a.k.a. mercury) gilding provides a robustly uniform coating of precious metal over the whole of an object. It works particularly well on copper-based objects, including sterling (a silver-copper alloy), brass and bronze, though even iron can be gilded if the surface is properly prepared beforehand.

Smithsonian, licensed under CC0 1.0
How does this work? When a piece of gold is placed into liquid mercury, at room temperature, it dissolves to form an amalgam, as mentioned earlier in this piece. The proportions required for fire gilding are 1:8 parts gold to mercury (this ratio, still used to this day, was documented by ancient scribes). This mixture of metals forms a paste-like amalgam which can be spread like thick honey over the object to be gilded.
Heating at relatively low temperatures, usually over a fire, is used to drive off the mercury to leave a layer of gold on the object’s surface (mercury boils at 357º C, whereas gold boils at 2,970° C). The layer is then burnished, rubbed with a smooth stone to compact the gold, making it shiny and bright (traditionally agate is used for this). In fact, the heat also diffuses the gold into the surface of the underlying metal, making it tough and relatively hard-wearing. The process can be repeated, if required, to build up a thicker ‘skin’ of gold.
That mercury was viewed as an otherworldly substance, possibly magical, is clear too from archaeological sites in other areas of the world. In Mexico, at Teotihuacan, there stands a series of pre-Columbian pyramids, dating to c.400 BC. Under one of these, the Temple of the Feathered Serpent, were found a range of unusual objects: jade statues, the skeletal remains of jaguars, and a box filled with carved shells and rubber balls.
The Feathered Serpent is a god, or supernatural creature, representing storms and rain, who dates back to the Olmec culture (c. 1,400–400 BC) and was worshipped as a bringer of life and fertility, albeit a rather capricious one. Known by the Aztecs as Quetzalcoatl or Tlāloc, the serpent is linked to earlier deities like the Mayan ‘Chaac’ or Zapotec ‘Cocijo’.
In addition, in deep chambers under the pyramid, a variety of glittering, reflective objects such as pyrite mirrors, objects made with obsidian and mica, and metallic spheres dubbed ‘disco balls’, have been uncovered. The big surprise, however, was finding a large quantity of liquid mercury (making excavation just a tad tricky). Academic Annabeth Headrick, whose specialisation is the art of Ancient American cultures, proposes that this represents a river, a way into the underworld, calling it “not that different from the river Styx”. Given that archaeologists have found mercury at three other sites around Central America, and the fact that mercury may be seen as symbolic, perhaps a ritual substance, she may have a point.
To the alchemists, the proto-scientist natural philosophers, it was magical too. They believed there to exist five classical ‘elements’ (æther, air, earth, fire, and water), and three ‘principles’: sulphur (the stone which burns, representing the soul), saltpetre (or nitre, embodying the, er, body), and mercury which provided the ‘essence’ of metals (symbolising the spirit). Alchemists believed that by determining the perfect proportions of mercury and other ingredients they could transmute base metals into gold. Mercury was also seen as a vital component of a panacea (cure for all ills), and the ‘elixir of life’. This belief arose from the Vedas, early Indian religious texts which alluded to both immortality and gold being inextricably linked, since gold was seen as eternal (it resists tarnishing, or ageing—exactly what people would wish for).
I’ve mentioned the use of amalgamation, using liquid mercury to ‘mine’ or separate gold from other materials. Well, this too has a long history. The earliest known use of this technique comes from the ancient city of Sardis, capital of the ancient Lydian Empire in what is now Turkey. Sardis was the home of King Croesus, renowned for his immense wealth, and the region has long been called the cradle of metallurgy.
There, from around 700 BC, deposits of alluvial or placer gold were known to be collected from the riverbeds of the Pactolus (where it had been washed downstream and deposited). Being some distance from the source in the mountain range to the south of Sardis, any gold would be in the form of fine-grained, flattened flakes. Not easy to collect by panning, and difficult to separate from the gold-bearing mineral mass known as ‘black sand’.
The Greek philosopher Strabo described the way that gold could be trapped by submerging sheep fleeces, stretched on a wooden frame, in a stream (the origins of the Golden Fleece myth). Flecks of gold carried downstream would collect in the lanolin-rich, greasy fleece. The fleece could then be left to dry, and the gold shaken or combed out. Whilst possible, and almost certainly used, this is relatively wasteful and inefficient method since it cannot guarantee that all the gold is retrieved nor actually separate the precious gold from other heavy minerals. To selectively remove only the gold from the black sand, researchers have shown that there is strong evidence that the Lydians used mercury (readily available in the locality) and amalgamation to accumulate their untold wealth.
Similar processing methods have been recognised in other parts of the world, including by the Chavin and Moche cultures in northern Peru, with amalgamation techniques dating to c.100 BC being identified. The Romans certainly knew that gold and mercury would amalgamate. So far, no evidence (written or practical) demonstrates that they actually made use of this process. Indeed, the earliest documentary account of mercury being used to ‘mine’ alluvial gold comes considerably later, from the 11th century Persian scientist and polymath Al-Biruni (Abū al-Rayḥān Muḥammad ibn Aḥmad al-Bīrūnī), an extraordinary man and a contemporary of Avicenna (Ibn Sīnā).
We’ve already seen that mercury and its compounds are toxic when deliberately ingested in the form of medicines, but what of accidental absorption? Well, that has long been something of a problem too, and across a number of different industries.
Mining is the obvious one. Inhaling particles of mercury-bearing ore is an understandable problem. The Romans were well aware of this. Nice chaps, they used their mines as penal colonies, sending criminals and ne’er-do-wells there to work and die. Not only did this get the work done for minimal expense, but if poisoned prisoners perished, it saved on execution costs too.
High levels of mercury were detected in bones from the Late Neolithic to Middle Chalcolithic, near the Almadén mines in southern Iberia. Whether this was from the practice of mining or subsequent use of the pulverised cinnabar is, however, the subject of some debate.
Mercury (or rather, cinnabar) mining’s not great for the environment either. No aspect of the Almadén district has escaped high levels of mercury contamination. The rocks, soils, and sediments for miles around all show elevated levels, as do waterways, and the flora and fauna. Heavy mercury pollution from mining dating back as early as 1,400 BC has also been documented around the mines at Huancavelica, in Peru (which the Inca called la mina de los muertos, the mine of death). Whether this pollution stems from merely extracting the cinnabar ore (for use as a pigment), or from ore processing to yield liquid mercury on site is unclear.
But it wasn’t only mining, as other industries used mercury in quantity, and their workers will also have suffered the consequences of contact with this toxic substance. The use of mercury amalgams in gold extraction and fire gilding will doubtless have caused significant health problems for huge numbers of people over hundreds of years. The scale of this cannot really be calculated.
More recently though, a colloquial English term for being a bit doolally is ‘mad as a hatter’. This is thought to derive from the behavioural changes seen in hat-makers from the use of mercury in fur felting, a complicated process. Long-term exposure to mercury vapour led them to develop a characteristic neurological disorder, brain damage otherwise known as Mad Hatter’s Disease or erethism.

Public Health Image Library, public domain
It was Huguenot workmen, fleeing religious persecution In the 17th century, who brought a practice called ‘carroting’ to England. This used mercury nitrate to loosen fur fibres from animal skins. The fur was matted into felt by pummelling and boiling it. Mercury vapour is given off, even at room temperature, but with the heat from boiling the fumes were intensified.
Previously, urine (often camel, or that of the hatmaker) had been used for this purpose, so why the shift to using mercury? The method seems to have been discovered by accident when a syphilitic hatter (being treated with the delightful medicaments we explored earlier) used his own mercury-laced urine and found that this produced a very high quality felt. This rather surprising advance was recognised, and the chemical adopted for use. It gave the fur and pelts a characteristic orangey colouration, hence the name.
Mercury, though perhaps not the safest element to play with, has found a plethora of other uses throughout its history. It could be found in the ‘silvering’ on the backs of mirrors, in paints, and was used in papermaking. Large baths of this liquid metal were used to float huge lighthouse lenses and mirrors, giving them low friction ‘bearings’ on which to rotate. It is used in batteries, float valves, relays, and electrical switches. It is used in LCD screens, ultraviolet (UV or blacklight) and fluorescent lamps.
Another mercury compound, mercury fulminate, Hg(CNO)2, is explosive. This tricky, unstable substance was used as a primer in firearms, as a trigger for other explosives in percussion and blasting caps, and detonators. The organomercury compound thimerosal, an antimicrobial, is one you may well have heard of in the last few years. It’s found in many vaccines (actually no, not those ones), but is also used (in small quantities) in contact lens solutions, some cosmetics (notably skin-lightening creams), and tattoo inks.
Mercury was, of course used in thermometers, and in sphygmomanometers (to measure blood pressure), but also in barometers. In 1643, Galileo suggested to his student, the Italian physicist Evangelista Torricelli, that he might use mercury in the experiments he was conducting to explore atmospheric pressure and the source of a vacuum. This meant that he could take advantage of a considerably shorter tube than he had been using. His first barometric experiments had been conducted using water. This required an approximately thirty-two-foot-long tube, an oddity which spawned considerable speculation that he was involved in witchcraft by his suspicious neighbours!
Torricelli took Galileo’s advice, filling a two-cubit glass tube (merely four feet long, or thereabouts) sealed at one end, with mercury, then inverting the tube into a dish filled with the metal. The mercury level at the top of the tube falls, leaving a gap (or vacuum space), but not because any mercury has escaped from the tube. In this way, Torricelli observed that a vacuum was created.
The level of mercury in the tube (the gap at the top) adjusts to match the atmospheric pressure pressing down on the mercury in the dish. As the atmospheric pressure increases, it forces a little more mercury up the tube, reducing the gap at the top. As the pressure drops, the gap becomes larger. By experimenting at different altitudes (taking his contraption up into the mountains), the height of the gap was seen to vary. A scale was added to distinguish the differing levels of mercury, and the barometer was born. The unit of measurement used for atmospheric pressures and for measuring high vacuum, the Torr, is (perhaps not surprisingly) named after Torricelli.

Adapted from Library of the University of Seville, licensed under CC BY 2.0 and Wellcome Collection, under CC BY 4.0
I can report with some pride that I have made a simple Torricelli barometer like the one in the diagram myself, using this element. Like those chaps in the cloaks and fancy hats, no I didn’t wear gloves (we didn’t back then) but I’m still here. Ah, but am I still sane? Who knows. I guess the jury is out.
Mercury is still used in many industrial processes and in scientific research, including in spectrophotometers (UV-Vis spectrometers), in liquid mirror telescopes, and as part of the most accurate atomic clock available as yet.
That said, on account of its toxicity, there’s a worldwide push to reduce the use of this element. Although mercury naturally enters the environment through the weathering process rocks undergo, and from volcanic activity, most of the release is seen to being due to the result of human activity. Despite technological advances to ‘clean up’ many industries, mercury pollution is said to be on the rise (although it’s conceivable that modern sampling and measurement techniques may simply highlight it, whereas previously it may have been there but unseen).
Mercury performs no known (as yet) biological role, but it can be found in every living thing. Most mercury, whether natural or anthropogenic, exists in an inorganic form, which isn’t a huge problem. However, it can be converted into organic mercury compounds (remember organic chemistry from the Carbon article?).
The driver for this is anaerobic microbes (typically aquatic, those which do not require oxygen to live). They convert mercury into methylmercury (CH3Hg+), which is more toxic at low doses than elemental mercury. Now part of an organic compound, it becomes ‘bioavailable’, so can be taken up into the tissues of organisms fairly readily. Thus, mercury (or its organic derivatives) enters the food chain.
However, the real problem is that it then ‘bioaccumulates’. This means that an organism (whether plant or animal) builds up higher concentrations of mercury than would be found in its surroundings, because the organism absorbs a substance, in this case mercury, faster than it can be eliminated.
In vegetation, mercury can be taken up from both the soil and water, and also from the atmosphere in which a plant photosynthesises and grows. Animals, however, cannot photosynthesise (to make their own food) so they eat plants, or chow down on other animals which have eaten plants.
This leads to ‘biomagnification’ (or bio amplification), where the levels of mercury increase as the tissues of one organism (e.g. plants) are consumed by another (a herbivore, such as sheep), which is then eaten by a carnivore or omnivore (er, us!). As you travel successively from lower to higher levels in a food chain, the concentration of mercury in tissues rises.
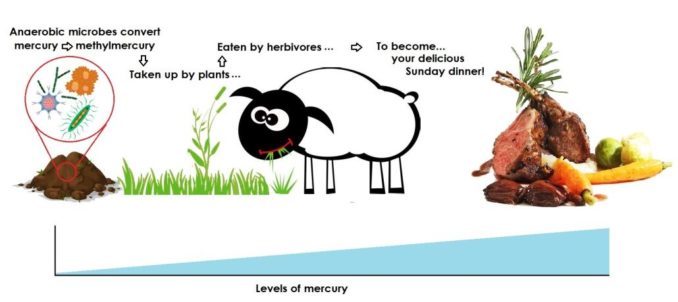
SharpieType301, 2023
This happens in aquatic and marine food chains too, with phytoplankton and algae at the bottom of the food chain being eaten by zooplankton. These are in turn eaten by shellfish and small fish, which become a snack for larger creatures, like seabirds, cephalopods and bigger fish. At the top of this food chain sit the big predators, like sharks… er, and us. It’s almost enough to put you off your scoff, eh?
Still, even given all this, we may not be the agents of our own demise. Good old planet Earth might yet do us in. Research, in part studying plant fossils from c.200 million years ago, has highlighted a process which they say ‘could’ have led to the mass extinctions that ended the Triassic period. The researchers proposed that high levels of mercury released by volcanic eruptions killed off animals. Some would have died from mercury poisoning, but if that didn’t see them off, a lack of food (as plants were also dying) would have meant they starved. They suggest that volcanic activity, and the release of mercury, could have played a significant part in four of the five mass extinctions over the past 600 million years.
The idea of mass extinction isn’t really a terribly cheery note to end on, so I thought I’d leave you with a conundrum. Perpetual or sepulchral lamps. Were they genuine, or merely mythical? Here are just a few examples which appear to fall into the ‘real’ camp.
In the early 5th century AD, theologian and philosopher Aurelius Augustinus (a.k.a. St. Augustine, the Bishop of Hippo) described in great detail an object found at an Egyptian temple, dedicated to the goddess Venus. This was a lamp that could not be extinguished by usual means. In his De Civitate Dei, he writes of “lucernam sic ardentem, ut eam nulla tempestas, nullus imber extingueret” (a lamp so burning that no storm, no rain could extinguish it). Augustine promptly denounced this as ‘magic’, condemning it as the work of the devil or ‘unclean demons’. Fascinating reading.
During the Papal reign of Paul III, between 1534 and November 1549, a tomb near the villa of Santa Maria Nova on the Appian Way was opened. This the burial place of Tullia (the beloved daughter of Cicero), who had died in 45 BC. The corpse was said to be covered in thick aromatic paste, upon removal of which the body was uncorrupted. An astonishing tale, particularly as “the whole of Rome, men and women, to the number of twenty thousand…” were said to have come to wonder at the sight of the ‘marvel of Santa Maria Nova’. As extraordinary as the body was a lamp. A burning lamp, which went out on exposure to the air, but which was believed to have burned in the sealed vault for 1,550 years.
A similar tale comes from Yorkshire, whose inhabitants are not usually folk to tell tall tales. At the time of King Henry VIII’s dissolution of the monasteries, a tomb near the Roman fort of Eboracum (York) was plundered. This was the tomb of Constantius I (Flavius Valerius Constantius ‘Chlorus’), who had died in July 306 AD. Constantius was the father of Constantine the Great, the first Christian emperor of Rome. In the tomb, it’s said that a lamp still burned, one which (if true) had been burning for more than 1,200 years.
Interestingly, with this tale the plot thickens. Constantius had re-conquered Roman Britain, from Allectus a Roman-Britannic usurper-emperor, who’d established a somewhat fleeting independent state in Britain. To mark this momentous event, Constantius had struck a medal bearing the phrase ‘Redditor Lucis Aeternae‘. Translated, this means ‘The Restorer of Eternal Light’.
So, could these everlasting lamps really have existed? Well, clinical psychologist Dr. Kathy Forti seems to think so, and indicates why she feels this is so on her website. The reason ties in with our element, mercury. That said, her name is uttered with similar reverence to that given David Icke, so I’ll leave you to make up your own mind.
The 17th century former Dean of St Paul’s, poet John Donne, obviously gave at least some credibility to these tales, as he referred to the legend in his poem written for bride Frances Howard, on the way to her marriage with Robert Carr, 1st Earl of Somerset.

Adapted from National Portrait Gallery, Public Domain
© SharpieType301 2023



