Here we go once again, embarking on our explorations of the elements. Up to now, we’ve considered Hydrogen, Carbon, Mercury, Bromine, Oxygen, and Iron. We’ve also had a quick trot around a little basic chemistry, and explored how the Periodic Table (a.k.a. the Periodic Table of the Elements) came into being.
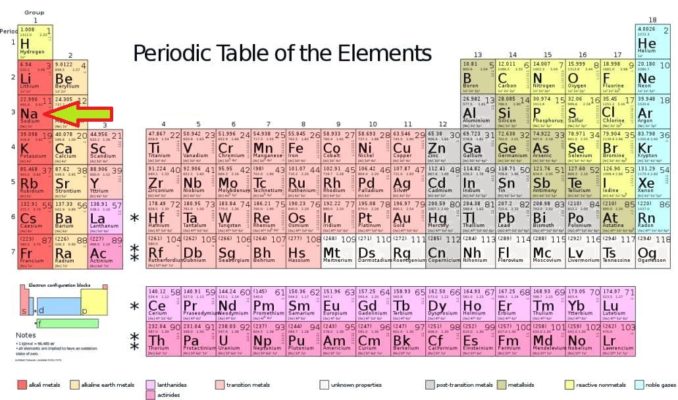
This time, we’re going to take a look at an element from the first Group of the Periodic Table, sodium.
2012rc, licensed under CC BY 3.0
Sodium was first isolated as an element in 1807, by Cornishman Sir Humphry Davy, with a pioneering technique using electricity to split compounds by electrolysis. The word ‘electrolysis’ derives from the Greek words ‘lysis’ (meaning ‘a loosening’) and ‘ēlektron’ (meaning ‘amber’, as static electricity can be generated by rubbing amber).
Davy had postulated that electricity was an essential property of matter, in effect the ‘glue’ that holds particles of matter together, so he proposed an ‘electrical theory of chemical affinity’. A further hypothesis was that when two elements combine to make a compound, electrical energy is released. So, by applying electricity to a compound, it should be possible to split the elements apart again.
To do this, he made use of a ‘voltaic pile’ (an early ‘battery’) developed by the Italian chemist Alessandro Volta, in 1799. This apparatus was made from alternating copper and zinc metal discs sandwiched between pieces of paper (or woollen felt), moistened with an electrolyte, a solution which conducts electricity—this was usually salt water, but acid was also used. Wires would be attached to the top and bottom of the stack. An example of a pile from this period can be seen below.

Wikimedia Commons, licensed under CC BY-SA 4.0
In 1800, remarkably soon after Volta’s apparatus was conceived, English scientists William Nicholson and Anthony Carlisle used it to break down water into its components by passing the current generated by a voltaic pile through it. The water decomposed by electrolysis into oxygen and hydrogen.
Davy was galvanised (pun, once again, intended) by these findings and built a huge version of this equipment in the basement of the Royal Institution. This ‘improved’ voltaic pile generated a sustained electrical current, which Davy used to break apart a number of common compounds.
One of these compounds was caustic soda, sodium hydroxide (NaOH), from which he isolated sodium, having just days before isolated the alkali metal element potassium from caustic potash. Enthused by this new technique, Davy observed that “Nothing promotes the advancement of science so much as a new instrument.”
His success prompted him to continue with his work on isolating the elements using electrolysis. In the end, he isolated not only sodium and potassium, but barium, boron, calcium, magnesium, and strontium.
It was Davy who coined the name ‘sodium’ for the element, as a hat-tip to the caustic soda used in his experiments. However, the chemical symbol, Na, comes from another chemist, Jöns Jacob Berzelius. It was he who, having repeated Davy’s experiments, chose this from the Latin word ‘natron’ for common soda (sodium carbonate).
They are unquestionably a lively lot, sodium, and it’s elemental relatives in Group 1 of the Periodic Table. Get them in contact with water and the fireworks will start, in some cases extremely literally. There are some wonderfully dramatic videos of these reactions out there in the wonderful world of the web, but my favourite has to be the one that British chemist Peter Wothers made for the Royal Society of Chemistry. To really see sodium in a starring role, watch it right to the end… Interestingly, even though we saw that sodium reacts quite explosively in contact with water, it will only burn gently in air.
Having seen this, you might be wondering why the Group 1 elements are such a reactive lot. Well, this is because all of them (lithium, sodium, potassium, rubidium, caesium, and francium) have just a single electron in their outermost electron shells.
Using our element sodium as an example, its atoms have 11 protons in their nuclei, thus 11 electrons. The first two electron shells are completely filled, with two electrons in the innermost shell, eight electrons in the second shell, and only one electron in the outermost shell.
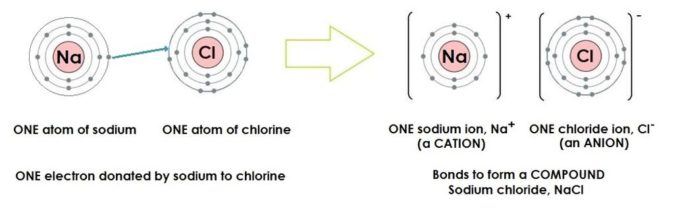
Sodium forms ionic bonds, by donating (some might say losing) that lone electron from its outer electron shell. Since it’s out there on its own, with that third shell far from being filled, it’s pretty easy for more ‘acquisitional’ atoms to pinch it.
This means sodium can form a wide variety of compounds, which can be either inorganic or organic. Sodium in compounds has an oxidation state of +1 (remember, the electron it loses leads to it having a positive charge).
SharpieType301, 2023
There are some other similarities between the Group 1 elements too. They have relatively low melting points (sodium melts at 97.8°C but only lithium, with a melting point of 180°C makes it into triple figures). They also have low densities, with lithium being the least dense of all metals. In fact, the density of the first three (lithium, sodium, and potassium) is low enough for them to float on water.
Being so reactive, sodium and the other Group 1 elements are usually stored under oil or, occasionally, a dry inert gas (e.g. nitrogen). The Group 1 elements are all soft, silvery-white lustrous metals that can be sliced easily with a penknife. When cut, the bright, shiny exposed surfaces rapidly dull and tarnish as they oxidise.
As do most metals, the Group 1 elements conduct heat and electricity easily. In the case of sodium, and its companion potassium from this group, electrical conductivity is very important to nerve function in the human body, something we’ll explore later.
Another interesting fact about them is that each element burns with a flame of a characteristic colour. This provides one way in which the individual elements may be identified (although unless the spectral lines produced are carefully examined, it’s more a strong hint than positive identification).
These colours form because the energy of the flame is absorbed by the metal as it is heated. This excites the electrons in the outer orbital (electron shell). They get pushed up into a higher orbital then, as that lone outermost electron drops back from a higher to a lower level (back to its previous, unexcited, position) it releases that absorbed energy as photons of visible light.
The colour of the light emitted depends on the energy required to move electrons from one orbital to another, and the colour emitted by larger atoms is lower in energy than the light emitted by smaller atoms. Lithium gives us a rich crimson red flame, sodium a striking yellowish-orange, potassium a delicate pinky-lilac flame, rubidium a reddish-violet, and caesium a blueish-violet flame.
As we have already seen, elemental sodium is less dense than water, so it floats… albeit rather briefly since it reacts vigorously with the water to produce sodium hydroxide. This is an exothermic reaction (one which releases heat), and one which also produces flammable hydrogen gas. In fact, the energy produced by the reaction causes the hydrogen to ignite and burn as the reaction takes place.
2 Na + 2 H20 → 2 NaOH + H2
The Group 1 elements are also called the alkali metals. They have been given this name because when they react with water, what is formed is an alkali. These are strong bases, which simply means that they are substances that can react with and neutralise acids. The word ‘alkali comes from the Arabic ‘al qalīy’, which refers to the ashes left when plant material is burned (these contain a residue of potassium hydroxide which, when immersed in water, creates lye (a corrosive substance used in making soap).
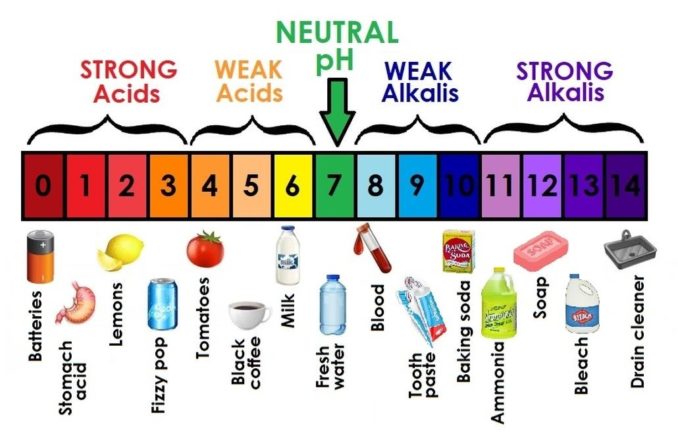
Alkalis dissolve in water to give a pH greater than 7 (a pH of 7 is neutral, and an example of a neutral solution is pure, fresh water). The alkalis will change the colour of an indicator (a chemical dye which changes colour at different pHs), for example turning the colour of ‘Universal Indicator’ to blue or purple. The alkalis have a soapy or slippery feel and, if you were feeling brave enough to try to taste them, they are bitter.
We’re going to take a little side-track here to look a little more closely at pH, and what it is. In essence, pH is a ranking, a scale, a spectrum if you like. That ‘p’ stands for potential, and the ‘H’ for hydrogen, so pH is the ‘potential of hydrogen’. As an analytical tool, what it does is allow the relative acidity or basicity of an aqueous solution to be precisely measured, by determining the potential of an aqueous solution to accept protons in the form of positively charged hydrogen ions (H+) compared to pure water. Once measured, a pH value is assigned.
The concept was developed in 1909 by the Danish chemist Søren Peter Lauritz Sørensen whilst studying the effects that ion concentration had on proteins. He called this phenomenon the ‘hydrogen ion exponent’ and gave it the notation pH.
We’ve already seen that a solution with a pH of 7 is neutral. What this actually means is that it is ‘balanced’ by having the same concentration of H+ ions and OH− ions, so is neither acidic nor alkaline.
Looking at the scale, any pH value below 7 denotes an acid (growing stronger the closer the value gets to 0) while a pH value above 7 indicates an alkali (becoming stronger the closer the value gets to 14). Nominally, the pH scale runs from 0 to 14, but it is possible to measure more extreme values outside these endpoints.
Whilst it looks as though it should be linear, the pH scale is actually logarithmic.
- A solution with a pH of 4 is ten times more acidic than a solution whose pH is 5.
- That same solution is ten times less acidic than a solution whose pH is 3.
The scale may look as though it has discrete values, but it is actually continuous, meaning it’s possible for a solution to be assigned a pH value at any point along the scale, e.g. a pH of 4.2, or 10.6.
The graphic below gives an indication (pun most definitely intended) of the pH values of some common everyday aqueous solutions.
SharpieType301, 2023
Back to our element, sodium, which is a relatively common element, in fact it’s the fifth most abundant metal on Earth, the most abundant alkali metal, and the sixth most abundant element overall. However, because it is so reactive, it is never found as a pure element. Instead, it is found in compound form.
The most common of these (comprising c. 80% of the dissolved components of seawater) is sodium chloride, also called halite. We know this best as table salt. Other common sodium-based minerals include the silicaceous feldspars, micas, and amphiboles (such as Na3Mg4AlSi8O22(OH)2 or Na2Mg3Al2Si8O22(OH)2), cryolite (sodium hexafluoroaluminate, Na3AlF6), nitratite (sodium nitrate, NaNO3), sodalite (which is Na8[Al6Si6O24]Cl2, rather than a low calorie fizzy drink) and zeolite (hydrated aluminium silicate, NaAlSi2O6-H2O).
Sodium has a body-centred cubic crystalline structure but has no allotropes. However, there are twenty-one isotopes of sodium. Of these, only 23Na, is stable.
As to the radioisotopes, 24Na can be created from 23Na by neutron activation, with powerful gamma rays being emitted as part of the decay. Gamma rays are electromagnetic waves with the shortest wavelengths. This makes them powerful and destructive. They can penetrate matter easily, not least human tissue. Indeed, they have such high penetration power, they can easily pass right through the body, causing immense damage to internal organs and other tissues at the cellular level inside the body (including disrupting our DNA) along the way.
For this reason, the emission of gamma rays as part of a nuclear reaction, sodium metal has been suggested as a casing material for nuclear weaponry to produce a ‘salted’ bomb. This fiendish concept is intended as a radiological weapon, deliberately producing extreme radiation poisoning through increased radioactive fallout. Such weapons, unsurprisingly, are classified as ‘weapons of mass destruction’ (WMDs, remember those?), as they can cause large tracts of land to become completely uninhabitable.
To provide a comparison, it has been calculated that a ‘standard’ 20-megaton* bomb could devastate an area of over 1,450 square miles. Note that a device of this magnitude would be *c.25% larger than the TX-21 ‘Shrimp’ nuclear gravity bomb. This device, nicknamed ‘Castle Bravo’, was unleashed at Bikini Atoll in 1954. It remains the most powerful thermonuclear bomb the United States has ever detonated.
However, if ‘salted’ with sodium, a device of the same size would produce enough radiation to contaminate a zone greater than the area of Spain, some 200,000 square miles. To my way of thinking, that’s quite some pinch of salt!
Speaking of salt, (or to give its chemical name, sodium chloride, NaCl), this substance has been of great value to many peoples in antiquity, and throughout history.
In hunter-gather times, the human diet was heavily meat-based. Red meat, particularly organ meat, is a good source of dietary sodium so although salt may have been used as an occasional flavouring, additional salt was not in truth an important requirement. However, with the rise of agrarian societies and a diet based around cereals and vegetables, there arose a need for additional salt in the diet.
A further reason for the importance of salt is that it offers one of the oldest known methods of preserving precious foodstuffs, particularly meat. Preservation allows societies to lessen their dependence on seasonal availability of food resources (the hunt being more likely to succeed at certain times of year) and maintain a more reliable, and possibly varied, diet year-round.
Salt (both the sodium and chloride parts) is an essential mineral for both humans and animals. Thus, natural salt licks (and rarer salt springs) would have been important to both throughout history, not least because, depending on the source, they also provide a raft of other trace amounts of essential elements. This is what gives the salts in the image below their distinctive colouration.

Larry Hoffman, licensed under CC BY 2.0
However, this precious resource was not necessarily available everywhere, particularly in inland areas. That very scarcity made the substance precious. With the gradual spread of civilisation and the rise of permanent settlements, salt became a valuable trade commodity.
The first site where salt was deliberately extracted not only for use by a small community but also for trade has been identified in what is now Romania. At Poiana Slatinei (in Lunca, Western Moldavia), at the foot of the Carpathians, salt production dates back to the early neolithic (around 6,050-5,500 BC BC). This area is rich in salt springs, some of which, at c.160 g/l, are much more concentrated than seawater. Here, ‘briquetage’ (remains of coarse clay vessels) is evidence that brine was boiled to concentrate and crystallise the salt into ‘salt cakes’. Once solid, the ‘cakes’ could be transported over long distances, and traded with areas deficient in this mineral.
Salt became so highly prized that ‘roads’, prehistoric and later trade routes, stretched across Europe and indeed across many parts of the world (quite a fun fact for a chemical compound that’s used to de-ice roads in winter). These routes ensured it became available to regions that were naturally lacking this precious resource. The trade in salt was highly lucrative, and precise trade routes often fell in and out of favour, shifting over time as the salt ‘taxes’ levied by towns along the way adjusted with changes in political conditions.
One well-known example of a ‘salt road’ is the Via Salaria, which crosses Italy from the salt pans at the mouth of the River Tronto on the Adriatic to the Imperial city of Rome. It was built by Sabines in the mid-3rd century BC and provided a guaranteed supply of salt to the citizens of Rome. Another is the Via del Sale, a centuries-old trade route used to transport Mediterranean salt from Nice up and over the Col de Tende (a high mountain Alpine pass mentioned by our friend Always Worth Saying in his recent piece ‘Through The Mountains To Piedmont‘) to the House of Savoy, in Turin.
Closer to home, there are a number of ‘Salt Ways’, important trade routes leading from brine springs at Droitwich, to other parts of the country. In fact, the suffix ‘-wich’ or ‘-wych’ in the name of an English town means it was once a source of salt.
Around 450 BC, the Greek ‘Father of History’ Herodotus discussed trade routes from oases in the Libyan desert which took advantage of this resource. He also speaks of ‘salt’ used at different levels of Egyptian mummification. Whilst the affluent underwent a complex process, one step of which involved packing body cavities with natron (νίτρον in Greek, nṯrj in Ancient Egyptian) to dry the corpse and prevent corruption, he reported that poorer people simply had their internal organs removed and their bodies left in it for 70 days. I suppose it must have done the job, but although ‘salty’, natron is not salt (NaCl) as we know it. It’s actually a naturally occurring mixture of sodium carbonate and sodium bicarbonate.
There are countless references to salt in the Bible. It is referred to literally, as a substance to be valued and offered to God as part of religious sacrifice (“with all thine offerings thou shalt offer salt”, Leviticus 2:13). But allusions to salt were also metaphorical, indicating worth, permanency, and purification. It was symbolic of faithfulness, or in a notable case, the lack of it.
When Lot and his family were commanded by the Lord to flee the iniquitous fleshpots of Sodom and Gomorrah, and ordered ‘look not behind thee’, Lot’s wife faltered in her faith and did not obey this instruction. Thus, she witnessed the destruction of the great cities with brimstone and fire, but her backward glance cost her dearly. Genesis 19:26 tells us Lot’s wife “looked back from behind him, and she became a pillar of salt”.

Silly Deity, Public Domain
By around 77 AD, the Roman philosopher Pliny the Elder had a few things to say about the value of salt too. In his thirty-seven-volume manuscript Naturalis Historia (Natural History), he exclaims “ergo, Hercules, vita humanior sine sale non quit degere, adeoque necessarium elementum est uti transierit intellectus ad voluptates animi quoque nimias”.
This translates approximately to: “By Hercules, then, life cannot be lived humanely without salt—it is such an essential substance that its name is transferred to the excessive pleasures of the soul too”.
As used today, the word ‘salary’ brings to mind periodic compensation (payments) made by employer to an employee for ‘work-for-hire’. However, originally, it had nothing to do with money, and everything to do with salt. Although Roman soldiers were primarily paid in coin, they also received a handful of salt in return for their service. This practice gives rise, not only to the term salary (from the Latin word salarium), but to the common expression ‘being worth one’s salt’.
That said, payment in salt well precedes Roman times. In the fourth chapter of the Biblical book of Ezra the Scribe, he refers to ‘the salt that we have eaten in the palace’ indicating that the recipient is in the emperor’s service. Ezra writes of the time of Artaxerxes I, the Achaemenid emperor who ruled from 465 to 424 BC.
Salt is such a ubiquitous substance that it has spawned a variety of idioms we often still use to this day, such as:
A covenant of salt – a Biblical term, which means a binding, long-lasting agreement. The binding, or permanent, part of this reference probably relates to the use of salt as a preservative.
Salt of the earth – a decent, honest, dependable person, representing the best of society. The Rolling Stones recorded a song about them in 1968, included on their album ‘Beggars Banquet’. The term is another Biblical one, this refers to the value of salt, and comes from Matthew 5:13, “Ye are the salt of the earth: but if the salt have lost his savour, wherewith shall it be salted? It is thenceforth good for nothing, but to be cast out, and to be trodden under foot of men.”
To salt the earth – a very different prospect, this is a practice employed in antiquity by conquerors (such as the Hittites and Assyrians) of rendering a city already destroyed by war unavailable for re-inhabitation. Spreading salt over the land was symbolic, a curse which ensured the city could never rise again. In the Old Testament, Judges 9:45 speaks of the king of Shechem judging the Israelites who dared stand against him, “And Abimelech fought against the city all that day; and he took the city, and slew the people that was therein, and beat down the city, and sowed it with salt.” Given that ‘the city’ in question was his own capital, Shechem, he was obviously pretty annoyed.
Take it with a pinch of salt – listen, but be a little sceptical of what you hear. This seems to derive from Pliny’s Naturalis Historia, describing the formidable King of Pontus, Mithridates’, antidote to poison, whereby any harmful effects can be diminished by taking ‘two dried walnuts, two figs, and twenty leaves of rue… plus a grain of salt’.
Salt it away – save or preserve for later use, usually relating to money. Again, this probably relates to the use of salt as a preservative.
Salt the books – a fraudulent record, or falsification of accounts to make something appear more valuable. This refers to the value of salt, being a prized substance.
Rub salt in the wound –make a bad situation worse, make someone feel bad, often deliberately. This is likely to come from the practice of rubbing salt into lacerations left by flogging, causing intense pain, thus intensifying an already horrible punishment.
Above the salt – people of high standing, the affluent, upper echelons, and conversely, Beneath (or below) the salt – inferior, the lower classes, those unworthy of a seat near the head of the table. First used in 1599 by playwright Ben Jonson in his satirical ‘Cynthia’s Revels’, these terms originate from a time when salt was a precious commodity, so the one and only saltcellar was positioned halfway along a long table – if you sat near it, closer to the master of the house, you were well regarded.
An old salt, or a salty dog – an experienced man, quite often an ornery character, usually a sailor. Procol Harum recorded a beautiful song about the fate they might meet on their 1969 album of the same name ‘A Salty Dog’.
Like a dose of salts – to act extremely rapidly and thoroughly. It probably relates to something being flushed out of one’s system, from the rapidity with which Epsom salts acts as a laxative.
Back to the salt mines – to go back to work, usually without much enthusiasm. This likely refers to those unfortunate individuals forced as prisoners to labour in the salt mines of various despots.
Put salt on the tail – to try to capture or hold something or someone. This comes from a verse in ‘Simple Simon’, a nursery rhyme, and an old superstition that sprinkling salt onto its tail feathers will mean a bird cannot fly away.

Adapted from Rawpixel Ltd and other Public Domain images
Salt has prompted a variety of other superstitions too. It is believed to be protective, to repel evil, ghosts or demons. Surrounding something with a circle of salt or laying a line of salt across a threshold is thought to provide protection from an evil that cannot cross the salt barrier. Salt was placed on the tongues of unbaptised babies in Mediaeval times to protect them, and Sumo wrestlers cast a handful of salt into the ring to banish evil spirits.
When given as a gift, it is believed to bring good fortune and prosperity to the recipient, for example, if salt is given to a someone when they move into a new home. Similarly, putting salt into your right-hand pocket before participating in a commercial transaction is thought to bring good (if a tad messy) luck. When it comes to other forms of good fortune, if given to a bride on her wedding day, salt was thought to bring her fertility.
However, there are some negative superstitions too. If you wish to be rid of an unwanted guest who simply won’t leave, sprinkle a little salt under their chair. This will make them uneasy, and eager to depart (though this might just be because they’ll regard you as a bit of a fruitcake!).
If salt is accidentally spilled, this means bad luck, or that the devil lurks behind you to take your soul. To remedy this, use your right hand to throw salt over your left shoulder, right into his eye!
The story of sodium is not simply restricted to common salt, sodium chloride; however, we will revisit its role in the human body in a moment.
As an element, sodium is used in its own right as a laboratory reagent, and in manufacturing products such as dyestuffs and perfumes, and other organic compounds (in this application, it is typically used as a reducing agent). Sodium has been used as a deoxidant in metallurgy and (again as a reducing agent) in the preparation of calcium, zirconium, titanium, and other transition metals.
It has found uses in the production of some polymers (e.g. nylon and synthetic rubber), and synthetic detergents. Although LED lighting is now taking over to a great extent, for a long time, the distinctive yellow-orange street lighting was based on sodium-vapour lamps.
As a liquid metal, sodium is used as a heat transfer fluid (a coolant) in fast nuclear reactors and is just beginning to find applications in concentrating solar power (CSP) thermal energy systems. As well as liquid sodium, the sodium-potassium alloy (NaK) is also used for this purpose.
Alloys of sodium with other metals have also found a number of uses. We’ve encountered alloys in previous articles, but just as a reminder, they are simply mixtures, with atoms of each different element intermingled rather than tightly bonded.
Alloys can, as well as sodium, include titanium, lead, mercury, tin, magnesium, copper, and zinc. In other alloys, sodium is often used as a modifier. Modifiers are sometimes used to enhance or adjust selected characteristics or properties (e.g. ductility or machinability, strength or hardness, stability or resistance to corrosion, electrical resistivity, or ease of welding) of a melt or the finished alloy.
All this said, sodium’s compounds, particularly its salts, have found considerably more uses than the metal itself.
It’s about time to take a look at the role (actually roles) sodium plays in the human body, so chemistry and biology will intermingle for a while in the complex dance which is the chemistry of us.
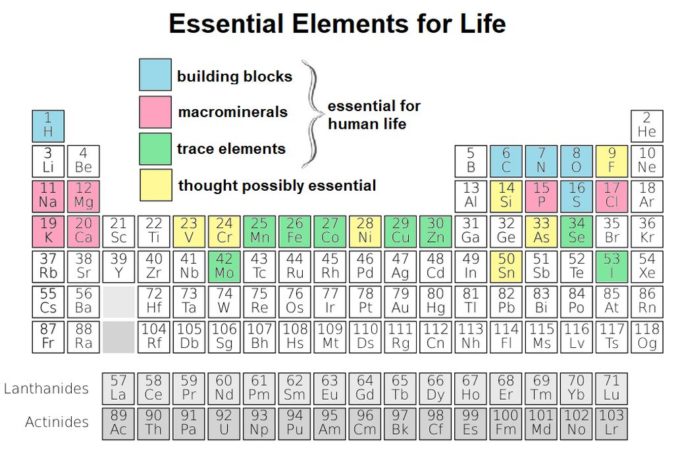
We have seen, in a previous article, the eleven elements (in pink and blue below) which are considered fundamental to life. Sodium, (Na), illustrated in pink, is one of these, and is considered one of the macrominerals. The macrominerals provide the essential ions required by the body and are primarily found in body fluids.
Sodium comprises about 0.2% by mass of the human body. Roughly speaking, that’s equivalent to about 100-150g, but the body gets a lot of bang for the buck with this relatively small amount.
SharpieType301, 2023, adapted from Cepheus, public domain
As we wander around in our daily lives, we humans are said to comprise somewhere in the region of 60% water. Pure water though? No. The importance of this watery fact is that all the chemical reactions of life take place in aqueous (water-based) solutions.
Most of this water, about two thirds, makes up the aqueous solutions that fill the body’s cells, called the intracellular fluids. The remaining third, known as extracellular fluids, surrounds all of the cells in the body. It is in these extracellular fluids that most of the body’s sodium can be found.
Although they make up only a third of our body fluids, the importance of the extracellular fluids cannot be overlooked. They make up the plasma in our blood, and lymph, and also the fluids which fill the cavities in our bodies. This is secreted by the serous membranes which clothe and line our internal organs (this fluid helps ensure they don’t stick to other body parts). Extracellular fluids are present in the cerebrospinal fluid that bathes the brain and spinal cord. They form the aqueous humour of our eyes, the synovial fluid in our joints. They make up the pleural fluid in our lungs. They are found in our muscles, and other body tissues. When you pick up a piece of meat and it feels moistly slimy to the touch, that’s extracellular fluid. It’s pretty much everywhere.
We’ve mentioned the essential elements. Well, some of the elements the body needs are electrolytes, that is elements dissolved in these fluids in the form of ions. The ions may have a positive electrical charge (cations) or be negatively charged (anions).
For example, sodium ions (Na+) are the most abundant cations in extracellular fluids, whereas potassium ions (K+) take this part inside the cells in the intracellular fluids. The primary anions are chloride ions (Cl–), which are the second-most abundant ions in our bodies. Other significant electrolytes are cations, magnesium (Mg++), calcium (Ca++), and anions, bicarbonates (HCO3–), and phosphates (H2PO4– and HPO4– –).
OK, so what use are they? Well, electrolytes (these essential ions) are indispensable for the basic functions of life. They conduct electrical charge, and maintain electrical neutrality, regulating the careful balance between positive cations and negative anions (electrically, most body fluids are neutral). The ions’ electrical charges also help drive the body’s chemical reactions.
These ions, including Na+, help buffer body fluids to maintain an optimal level of pH, the acid-base balance. In the human body, the average pH is slightly alkaline, ranging from pH 7.35 to 7.45 (though different organs require their own ‘optimal’ pH).
Ions, again including Na+, also help maintain a delicate balance of fluids within and outside the cells (this is essential to the control of our blood volume and blood pressure).
In addition, they help maintain the correct balance of minerals which, in turn, allows your body to control the nervous system, hormones in the endocrine system, and regulate how substances (nutrients and waste) are transported in and out through the semi-permeable membranes of cells—this is known as membrane permeability.
So, you’ll get the picture that electrolytes are pretty important. The consequences of an electrolyte imbalance are, like the whole subject, incredibly complex, but let’s just leave it that it’ll do you no darn good at all.
Let’s take a specific look at sodium’s part in all this. The two principal roles played by the Na+ ions are maintaining the extracellular fluid volume (osmoregulation) and maintaining the correct concentrations of Na+ and K+ in living cells. The second of these roles is key, as it dictates the performance of neurons (nerve cells) so that nerve impulses, electrical messages, are transmitted between body and brain.
Na+ ions also play a vital role in the way a number of enzymes function and, perhaps even more crucially, in the contraction/relaxation of muscles. Why so important, you might ask? Well, there are three types of muscle, all of which rely on sodium:
- Cardiac muscle – found only in the heart, these muscles form part of the heart walls and allow blood to be pumped throughout the cardiovascular system. These muscles are not under our conscious control, so are also called ‘involuntary’ muscles (sodium is involved in the signals in the heart which tells cardiac muscle when to contract, which it does rhythmically).
- Smooth muscle- these are also ‘involuntary’ muscles, so not typically under our conscious control, though there are some exceptions. These muscles line and support the organs, including the bladder, stomach, and intestines, and contract slowly and automatically. They are also found in the wall of blood vessels, and are the muscles used when we breathe, to expand the lungs.
- Striated, or skeletal, muscle – these are the muscles which, together with the bones, tendons, and ligaments, support the body and help us move. These muscles are under our conscious control, so are also called ‘voluntary’ muscles. There are sub-types to these, slow-twitch muscles, the aerobic muscles which are the body’s first choice for general use (e.g. those involved in posture) and sustained movement, and fast-twitch muscles which are employed where sudden bursts of energy are required (e.g. sprinting and jumping).
Without sodium and its ions, I think it’s clear to see we’d be pretty well snookered. But how is the ‘correct’ level of this precious element achieved?
We obtain the sodium we need from the food and drink we consume, but we also lose it from our bodies, chiefly in sweat and urine. Our kidneys (assuming these are healthy) are responsible for maintaining the required, consistent level of sodium in the body. This they do by adjusting the amount excreted in the urine. If intake ßà excretion gets out of kilter for some reason (e.g. underlying illness or the effects of some medications), the total amount (level) of sodium in the body is affected.
This can lead to hyponatraemia – having too much fluid and not enough sodium, or hypernatraemia – having too much sodium and not enough fluid. Oddly, both of these different conditions can result from becoming dehydrated. Both are treatable, but of the two, hypernatremia is the more problematic.
If not diagnosed correctly, and treated properly, hypernatraemia can lead to permanent brain damage, or be fatal. It particularly affects older individuals, since their ability to sense thirst may be impaired. It can also be triggered by deterioration of kidney function, or by the hormones regulating the salt : water balance not working as they should. Since many of us are beginning to feel the passage of the years, it’s rather a cheerful thought…
I can’t possibly end this piece on such a downbeat note, so I’ll leave you with a thing of beauty. This piece is the only gold sculpture by the talented Florentine goldsmith and sculptor, Benvenuto Cellini still in existence. So important is this piece, it has been called the ‘Mona Lisa of Sculpture’. It is… a salt cellar!

Jerzy Strzelecki, licensed under CC BY 3.0
Dating to 1543, the incredible Cellini ‘Saliera’ was made for the King of France, François I, a great patron of the arts. At just ten inches in height, it stands as a spectacular object in its own right (practical too), but its value is enhanced by the copious notes Cellini made about the construction of such sculptures. These can be found in his I trattati dell’oreficieria e della Scultura. The primary material is gold, but contrary to what might be expected, this is not a mould-cast work, but carefully hammered to sculpt it by hand.
The male figure on the right is Neptune, the Roman god of the sea (the trident is his symbol). He reclines next to a ship, sadly hidden by his knees in the image, which is the receptacle for the sea’s treasure, salt. The woman facing him is Tellus, also called Terra Mater, the ancient Roman goddess of the Earth. Tellus reaches down to indicate a miniature temple by her feet, which denotes Earth’s bounty. This would have held peppercorns. Their feet are entwined, to show the close relationship of the land and the sea.

Joanbanjo, licensed under CC BY-SA 3.0
The top of the oval base (see above) is an absolute delight, exquisitely decorated with gloriously rich coloured vitreous enamelling and tiny ivory details: fish, shells, sea horses and other marine creatures. The sides panels show the classical elements (earth, water, air and fire), and the winds (so important to mariners) as cherubs with puffed cheeks, blowing the winds in the four cardinal directions. There’s symbolism galore in this work, as an allegory of the Cosmos. It is set on an ebony base which has bearings underneath, allowing it to be rolled across the surface of a banqueting table to share its bounty.
It currently resides in the Kunsthistorisches Museum in Vienna. But, if it ever goes missing (er, again, it was stolen in 2003, but thankfully found) you might look for it wherever I am.
© SharpieType301 2023



