In previous articles, we’ve looked at the element Hydrogen, and at the development of the Periodic Table (a.k.a. the Periodic Table of the Elements).
This time, I intend to shine the spotlight on an element that some say is the most interesting of all of the known elements. It’s one which is fundamental to our very existence, Carbon.
Modified from 2012rc, licensed under CC BY 3.0
Carbon is a truly fascinating element. It is the fourth most abundant element in the universe, with only hydrogen, helium, and oxygen (in that order) being more abundant. It is created by nuclear fusion in stars (giant and supergiant stars, particularly red giants), derived from helium, via the triple-alpha process.
It’s believed that, on Earth, there are more molecules which contain carbon than any other element. Just think about that for a second. Take a look around you. Whatever your gaze happens to fall upon will quite likely contain this element. The very fact that you can see at all is down to carbon being a fundamental component of the physical structure which is your body, and an intrinsic part of the reason it functions.
This abundance is because carbon atoms appear as one ‘ingredient’ in a plethora of different compounds: compounds, which we’ve encountered before, being molecules made up of combinations of several elements. The reason for this is that, because of the way carbon atoms bond to other atoms, they are able to form chains really easily. We’ll see more of this later.
So, carbon-based molecules obviously contain carbon, but typically as just one part of their elemental composition. Many materials we make use of on a daily basis, things like our food, pharmaceutical products, plastics, etc., are carbon-based.
This one element, and the molecules it can form, has given rise to an entire subdiscipline of chemistry. This is organic chemistry, a subject which is dedicated to the study of the chemistry of life but also of all compounds which contain carbon atoms. Just on that basis alone, carbon really ought to have a series of articles all of its very own, but that’s a job for another day.
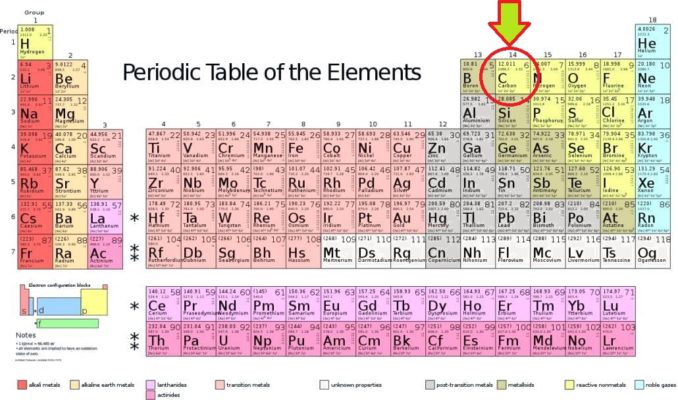
As you’ll see from the Periodic Table above, carbon lies in Group 14 (a.k.a. the tetrels). Its family includes the elements silicon (Si), germanium (Ge), tin (Sn), and lead (Pb). At the foot of this group is an extremely radioactive synthetic element, flerovium (Fl), only discovered in 1999, and of which only about 80 atoms have ever been identified.
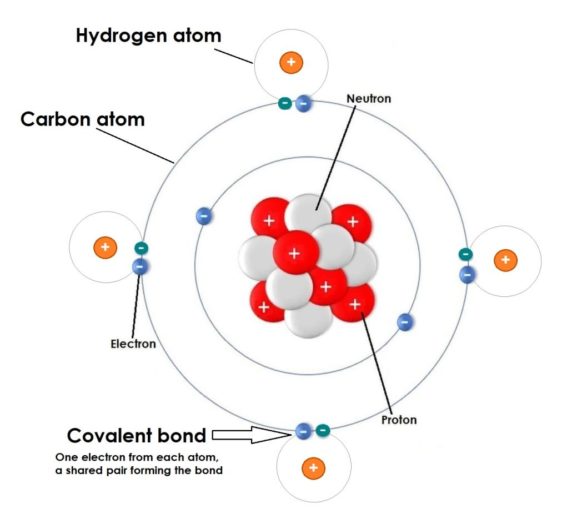
Carbon is a non-metal (although part of a family which includes metals). Carbon has been given the symbol C. Its atomic number is 6, as each carbon atom has six protons in its nucleus. Carbon is a tetravalent element (as are all in Group 14), which means that each carbon atom has four electrons ready and willing to form covalent chemical bonds.
SharpieType301, 2023
A covalent bond can be thought of as a nicely egalitarian, ‘carey sharey’, consensual sort of arrangement. Atom A and atom B form a chemical link between them whereby a pair of electrons (one contributed by each atom) is shared between them. Snuggly, eh?
This contrasts with the ionic bond, a rather less altruistic type of bonding. This category of bond occurs when one atom (typically one of the non-metal elements, cheeky little beggars) essentially ‘makes off’ with an electron from its partner’s electron cloud (normally a poor, unsuspecting metal). It ‘steals’ this electron, drawing it away from its partner to fill its own electron cloud in order to complete it.
With ionic bonding, the sneaky ‘pickpocket’ element becomes negatively charged (an anion), leaving the other atom positively charged (a cation). I’ll provide a bit more detail when we eventually talk about some of the metal elements but, for now, it’s not relevant. Our friend carbon, being Mr Nice Guy, doesn’t do this.
When we looked at hydrogen in a previous article, I mentioned that elements often exist with more than one isotope (where the numbers of protons found in the atom’s nucleus remains the same, but the number of neutrons differs). Each isotope is still the same element, so each will exhibit chemical properties that are very similar to the others.
Carbon is no exception to this and has quite a few isotopes. Fifteen of them, in fact, these being: 8C, 9C, 10C, 11C, 12C, 13C, 14C, 15C, 16C, 17C, 18C, 19C, 20C, 21C, and 22C.
Of these, only the isotopes 12C (with 6 protons, 6 neutrons), and 13C (with 6 protons, 7 neutrons) are stable. It is only these two stable isotopes, plus 14C (which has 6 protons and 8 neutrons) that occur naturally on Earth. 14C, also called radiocarbon, is a radioactive isotope with a half-life of 5730 ± 40 years. It is the presence of 14C in trace amounts in organic materials which forms the basis of radiocarbon dating, to which we will return a little later.
Although a single element, albeit one with a variety of isotopes, carbon can also exist in a number of different allotropes. Oh boy, we’ve barely got started and the woman’s at it again. Now what the heck is an allotrope?
Well, the term allotrope is used to describe an element which exists in more than one crystalline or structural form within the same physical state, or phase (note: structure is an important concept in chemistry). Specifically, in relation to carbon, this is the solid phase (for other elements, although less commonly, the phase could be a liquid or gas). ‘Allotrope’ was coined by the Swedish chemist, Jöns Jacob Berzelius, in 1841, combining words from the Ancient Greek: ἄλλος, meaning ‘other’, with τρόπος, which means ‘manner’ or ‘form’.
Remember carbon atoms sharing their electrons to form covalent bonds? Allotropes are possible because of the different ways the in which atoms of that element can bond, one to another. Because of this difference, each allotrope can display chemical and physical properties that are quite distinct from one another.
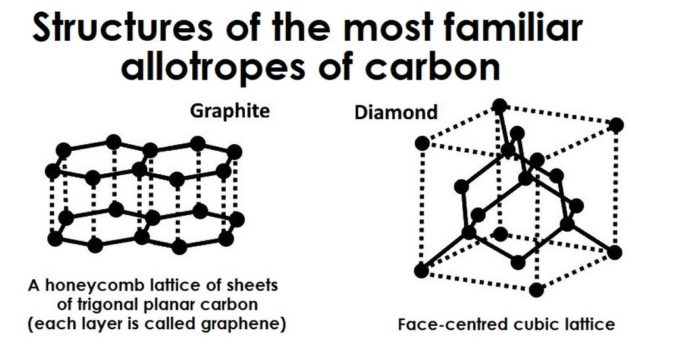
The allotropes of carbon which are probably most familiar to us are diamonds (which, thanks to Shirley Bassey, we all know are forever) and graphite. However, these aren’t the only ones: we will touch on some others later.
Just as an aside, how do we know that a diamond is actually a form of pure carbon?
In previous articles, we’ve encountered French scientist Antoine-Laurent de Lavoisier. In 1772, he performed an experiment whereby he set up a diamond in a sealed glass jar, filled with oxygen gas. For this experiment, he used a ‘burning lens’ to concentrate the sun’s rays onto the diamond’s surface. The ‘burning’ lens was first developed by German scientist Ehrenfried Walter von Tschirnhaus. It was, in effect, a series of high-quality magnifying lenses, positioned to focus the sun’s rays to their finest possible point, thus generating intense heat.

Daderot, public domain
The one shown above is a relatively small table-top instrument, but Lavoisier employed the ‘Great Burning Glass of Trudaine’. This apparatus was huge: two glass spheres, filled with alcohol, each 8 feet in radius, placed 4 feet apart. Beyond this lay a solid glass auxiliary lens, which focused the sun’s rays to a focal point less than an inch across.
Given this thermal onslaught, perhaps unsurprisingly, the diamond burned (he’d already debunked the theory that phlogiston was responsible for combustion), vanishing completely. Lavoisier documented that, although the diamond had decreased in mass, the overall weight of the jar was unchanged. This observation was significant, demonstrating that the new underlying law of nature he proposed, the ‘law of conservation of mass’, was correct.
His experimental results indicated that the diamond had been turned into a colourless gas. He performed the same experiment using charcoal, with the same result. Since all that was contained in the jar was oxygen and the material being burned (diamond or charcoal), both of these must made from the same substance, in different forms. He gave this element the name carbon.
The gas produced in each case was carbon dioxide, CO2 (then known as ‘fixed air’). This could be shown by bubbling it through limewater, turning the liquid a milky, cloudy white.
Diamond and graphite are both crystalline forms of carbon, where atoms are arranged in an ordered, regular structure. The other allotrope found freely in nature is amorphous (non-crystalline, disordered) which you may know in the form of lampblack.
Adapted from Diepizza, licensed under CC BY-SA 4.0
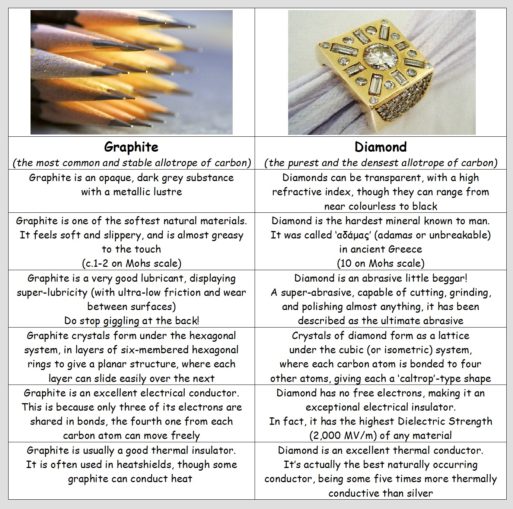
As these two allotropes are the same element (carbon), they share some similarities. Both exhibit a giant covalent structure (which sort of means there are no limits to the size they can achieve, simply building a smaller or larger macromolecule). Both have a high melting point and are extremely chemically stable and resistant. However, their other properties are demonstrably different.
SharpieType301, 2023, with images from cygnus921 and MAURO CATEB, both licensed under CC BY 2.0
The element carbon has been known to and been used by man for a very long time: in the form of charcoal, back to early prehistory. Indeed, the element’s name is derived from the Latin carbo ‘burnt wood’.
In the form of graphite, carbon was used by the Boian culture (around 4,300–3,500 BC) of south-eastern Europe to decorate ceramics. As a chemical addition to ceramics, a refractory additive, rendering it fire-proof (making use of those excellent thermal insulation properties), graphite was mined by the Late Bronze Age/early Iron Age Hallstat Celts in Southern Bohemia (modern day Czech Republic). From the oppidum of Třisov (oppida = a large, fortified Iron Age settlement), the ceramics produced, which had graphite added to the clay, achieved a very high level of technology. These products were traded far and wide.
A graphite mine (earliest known use is debated, possibly c.500 BC) on the outskirts of Český Krumlov was found to have particularly rich deposits. Some layers were up to 20 metres deep. This region remained an important mining area (producing not only graphite, but silver, gold and other metals and minerals) through the Middle Ages, and later. Now a tourist destination, graphite mining continued here until 2003.

James St. John, licensed under CC BY 2.0
Here in Great Britain, we have our own rich deposits of graphite (a.k.a. plumbago), near Keswick in Cumbria. This was used for the ‘lead’ in pencils, and as stove black (which you may know as ‘black lead’). In days gone by, Borrowdale graphite also made a significant contribution to the strength and effectiveness of our navy.
In the late 1500s, under the reign of Queen Elizabeth I when Sir Francis Drake was ‘king’ of the seas, the refractory nature of graphite was put to lethally effective use. I’m rather hoping our good friend Tom Pudding of this parish might be able to add more to this, but cannonballs, at this point being cast from iron, had a bit of a problem. They were rough-cast and irregular, and they rusted. To put it mildly, this meant that they were not all that consistent when fired. A bit of a beggar when one’s navy was surrounded by salty water. No-one likes rusty balls.
So, graphite from Borrowdale, known locally as ‘wad’ was introduced as a liner for cannonball moulds. This greatly improved them: they could be made rounder and with smoother surfaces (I also suspect, but cannot find confirmation, that remaining deposits of graphite on the iron surface made them less susceptible to rust). This meant that they were less likely to jam, flew straighter and could be fired greater distances.
This development led to something of an ‘arms race’ and made the graphite deposits of Borrowdale a very valuable resource indeed. Elizabeth I ensured this mine remained strictly under Crown control, and was only accessible via a guardhouse, passing armed guards. In fact, once enough supplies had been extracted, the mine was flooded to prevent access until more was needed. It’s alleged that the theft of this mineral (not least used by locals to mark their sheep!) may be the origin of the term ‘black market’. Though miners were searched at the end of each working day, anyone entering the mine without permission or caught stealing or trading precious graphite could be punished by hard labour or seven years transportation.
In more recent years, graphite has been put to a variety of uses in batteries, in the production of steel, and in brake linings and lubricants. However, a more commonplace but perhaps significant use was as the ‘lead’ in pencils, which impacted education and the transfer of knowledge for centuries. Though they later had ‘leads’ made from a mixture of clay, wax and graphite, the first pencils used a solid core of Borrowdale graphite, so this tiny locality in England cornered the market in these useful implements.
When it comes to carbon in diamond allotropic form, evidence of first use comes from around the same period. The Chinese Liangzhu and Sanxingcun cultures (which lie west of the modern city of Shanghai), dating to around 4,000-3,800 BC used small diamonds, more or less diamond dust, as an abrasive to grind and polish the extraordinarily hard corundum-rich stone for ceremonial burial axes, to a near unbelievably smooth surface.
The first written reference to diamonds comes from India, where the stones were noted in the Arthasastra, a 4th Century BC Sanskrit accounting book. They were, at this time being traded and taxed, but diamonds were known to be in use well before this as they’ve been observed in ancient Indian religious icons.
It has been established that, in 327 BC, Alexander the Great, brought the first diamonds to Europe from India. These stones were believed by the ancient Greeks to be the solidified tears of the gods. They were thought to have preservative and curative properties to ward off disease and repel malevolent spirits.
Other ancient civilisations also held them in great esteem. The Egyptians believed diamonds held mystical powers, and there’s a nice section in one of Wilbur Smith’s books where a character of presumed Phoenician origin describes the stones thus:
“When sun and moon show together in the sky, then it may happen that their rays mingle and become hot and heavy. They fall to earth, and if they strike water then they are quenched and freeze into one of these sun-stones.”
Fanciful fiction, perhaps, but I rather like it.
Plato postulated that the core of the world, or axis mundi, was a diamond. Actually, to some extent, he had a point. In fact, most diamonds are created deep within the Earth’s upper mantle, over 100 miles below the surface, when carbon-rich ores are compressed under extraordinarily high pressures and temperatures. They finally reach the surface, raised up by ancient volcanic eruptions. Given that, far from the rare stones we perceive them to be, it’s likely that they are relatively common deep in the core of our planet. It’s just that we mere humans have no access to them, thus making them precious to us.

James St. John, licensed under CC BY 2.0
A diamond’s value is dependent on size and colour. Blue-white diamonds are more valuable than those which show a colour, however minimal (though I must admit I’m partial to yellow and cognac stones). Some fluoresce under UV ‘black’ light, and others are phosphorescent, glowing with an inner light under certain conditions. Black diamonds are typically used in industry, not commonly in jewellery, and are therefore the cheapest.
The largest gem-quality diamond ever discovered on Earth, was found by pure chance. The Cullinan Diamond came from the Premier No. 2 mine’s Kimberlite pipe near Pretoria in South Africa, so probably appeared quite similar to those seen above.
Found in 1905, it was about the size of a fist, a whopping ten plus centimetres (over four inches) across at its widest point. The mining superintendent, one Frederick Wells, thought it a worthless chunk of crystal and it was very nearly discarded. Not really surprising since, uncut, it weighed 3026 carats, and weighed in at around a pound and a half. An extraordinary discovery.

via Wikimedia Commons, licensed under CC BY-SA 4.0
This massive, nearly colourless ‘blue-white’ diamond was not used in one piece. It was cut into almost a hundred different gems, by Joseph Asscher & Company of Amsterdam, in a painstaking and delicate process which took some eight months (and, I’d imagine, some serious sleepless nights).
The two largest cut diamonds from the Cullinan were set aside for King Edward. The greatest of these, the Great Star of Africa (or Cullinan I), at 530.4 carats, is incorporated into the Sovereign’s Sceptre with Cross, part of our Crown Jewels. Together with Cullinan II, the Second Star of Africa (a mere 317.4 carats in weight) which forms part of the Imperial State Crown, they are displayed at the Tower of London. Cullinan II, by the way, is the vast transparent sparkly rock just above the ermine trim, immediately below the Black Prince’s Ruby at the front of this iconic crown.

via Wikimedia Commons, public domain
But diamonds are not just an earthy delight. They can form wherever similar conditions are found, and some of the largest diamonds are believed to come from space. The white dwarf star BPM 37093 (V886 Centauri) is thought to be, in part, composed of crystallised carbon: diamond by any other name. This star was nicknamed ‘Lucy’ after the Beatles song, ‘Lucy in the Sky with Diamonds’. We’ve already seen that diamond, like graphite, has a giant covalent structure, meaning that it is possible for very large diamonds to form. Well, ‘Lucy’ is a big girl. Massive, in fact, at around 4000 kilometres in diameter, thus likely weighing in at c.10 billion trillion trillion carats. That’s quite some valuable rock!
Before we move on to carbon in living organisms, it’s worth a look at some of the element’s other allotropes. As we saw in Figure 3, a single layer of graphite is called graphene. Although, in theory, this allotrope was known about back in the late 1940s, it’s isolation is relatively recent. Two Manchester physicists, Andre Geim and Konstantin Novoselov, must take the credit… as does a roll of Sellotape!
Almost as Friday night joke-science, they stuck the tape onto some graphite and peeled some away the top to try to isolate a single layer. They folded the tape over in half, opening it to peel away a thinner layer, and went on to repeat this some 10, 20 or more times. Each time they got a thinner and thinner flake, until they had a layer just one atom thick! They had isolated graphene. OK, I must confess that this is only partly true. It was, in truth, slightly more scientific towards the end, but it’s a great story, and one for which they were awarded the 2010 Nobel Prize for Physics.
Why was this so important? Well, it allowed the extraordinary properties of this carbon allotrope to be explored. Graphene, at just one atom thick, is the world’s thinnest material. In proportion to its thickness, it is also the strongest and also the most conductive, both electrically and thermally. Dubbed a super-material, it’s potential for commercial applications was recognised almost immediately. I won’t go into detail as that would take way more than one article, all on its own, but graphene may yet revolutionise a great number of industries. If you’d like to learn more there’s a website HERE that’ll tell you much that you might wish to know.
These one-atom-thick flat graphene sheets can be manipulated to form another group of allotropes: the fullerenes. Again, these materials are pure carbon with no other elements included. The carbon atoms are mainly linked to one another by single bonds, but there are also some double bonds. This means that, as well as the hexagonal rings of graphene, some of the rings are pentagonal. This small quirk allows their structure, in effect the shapes they form, to curve.
Examples of these can be seen below. They include graphene nanoribbons (GNRs), essentially narrow strips of graphene which are categorised by the shapes of their edges, carbon nanotubes (CNTs) which can be either single- or multi-walled with one tube inside another, the so-called ‘buckyballs’ (actually spheroidal, fused-ring structures which look a bit like footballs), and carbon nanobuds (CNBs), sometimes referred to as ‘buckybuds’, which combine tubes and balls.
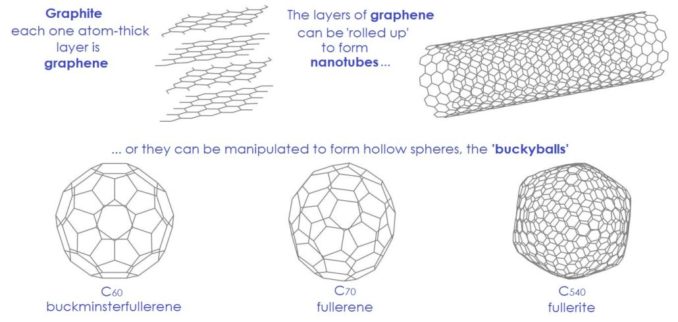
Adapted from Andel, licensed under CC BY-SA 4.0
The properties of the fullerenes is the subject of a huge body of research as, like graphene, their potential for commercial applications is immense.
As we’ve seen from the allotropes above, carbon is able to form chains really easily, to form molecules with a variety of different structures, and to form extremely large molecules. These properties are all extremely important concepts for the next area of carbon (a.k.a. organic) chemistry we will explore: the chemistry of life.
Every living organism is made up of biomolecules (a truncated term for biological molecules). These biomolecules are crucial to all biological processes, that is, to life, and… every single biomolecule contains carbon!
Although the exact chemical composition of living things (animals, plants, fungi, algae, and microorganisms like bacteria) differs slightly, let’s focus on people. Something like 96% of our human body comprises just four elements. These are our current element of interest carbon, plus oxygen, hydrogen, and nitrogen. The remaining 4% is a hodgepodge of other elements, including phosphorus, sulphur and chlorine, and a number of ‘biometals’, primarily calcium, potassium, sodium, and magnesium, although there are many more elements which form part of us, present only in trace amounts. By the way, the reason we are more oxygen than anything else, is that the human body comprises approximately 60% water (H2O).

Adapted from Willis Lam, licensed under CC BY-SA 2.0
At the most fundamental level, atoms of these different elements combine to form molecules, often with quite complex shapes. The ‘recipe’ for a molecule, i.e., its elemental composition, and the unique three-dimensional shape a specific molecule will take, determines its function.
So, what classes of molecule are these biomolecules? Well, they are the Lipids and Carbohydrates, which only contain carbon, hydrogen, and oxygen, the Nucleic acids, which also contain nitrogen and phosphorous, and the Proteins which also contain nitrogen, sometimes sulphur.
Nucleic acids are the building blocks of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). Each of these are large molecules, macromolecules. They are polymers, that is to say chains, made up of repeated small sub-units (monomers) bonded together. These sub-units are the nucleotides, which are molecules of three distinct parts which, when combined, allow the long chains (polymers) to form.
The nucleotides comprise one of five nucleobases (nitrogenous base compounds): adenine (A), cytosine (C), guanine (G), thymine (T – only found in DNA), and uracil (U – only found in RNA). Anyone thinking those letters sound a little familiar might be remembering the film GATTACA. To this base, is connected a five-carbon sugar (a pentose, either ribose or deoxyribose), and a phosphate.
Because of the way the chemical bonds are formed, the polymers built up from nucleic acids (RNA and DNA) create a distinct structure. This structure is the familiarly beautiful helical ‘strands’ of RNA and DNA we see below.
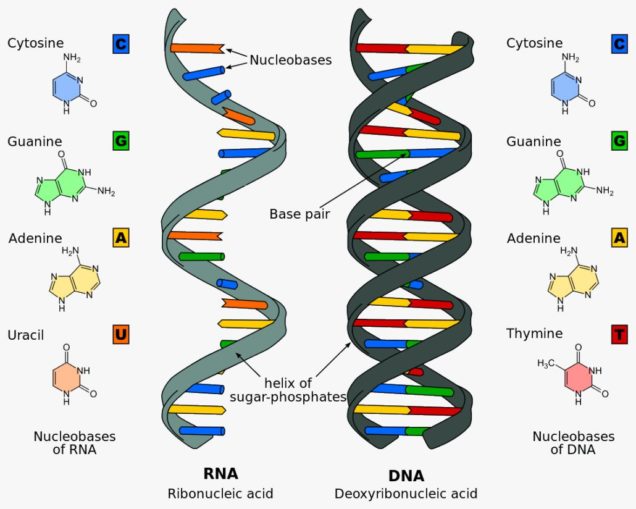
Sponk, licensed under CC BY-SA 3.0
DNA is a pretty big molecule (if you unravelled the DNA in just one of your cells, and laid it end to end, it’d be about six and a half feet in length!). It’s responsible for replicating and storing genetic information (a genetic code) in the nucleus of your cells. You could describe it as a ‘blueprint’. DNA therefore acts a bit like a biological USB drive which holds and guards the rules which enable the growth and development of organisms.
It tag-teams with RNA, which typically has much shorter strands than DNA. The RNA ‘reads’ these stored instructions and executes them to construct all of the proteins which go into the make-up of each living thing.
There are three main subtypes of RNA (acting like team-mates on a production line) which work in concert to do this:
- messenger (mRNA), that you’ll have heard a lot about in recent years, which translates the information from the DNA, and gets the ball rolling
- transfer (tRNA), which acts like the orchestra’s conductor
- ribosomal (rRNA), in effect the Doozers of the RNA world, who are responsible for the grunt work
There’s a fourth subtype too, microRNA (miRNA, which plays a part in regulating this activity). This process is known as gene expression, and it is an incredibly important process, one that’s utilised by all known life. None of this is possible without carbon.
The main commodities that come off this genetic production line are proteins. In animals, like ourselves, they perform a multitude of different roles. It’s proteins which provide the structure and function of all of our cells. They are crucial to good health, repairing and building body tissues. Proteins give us enzymes and hormones. They catalyse (initiate) chemical reactions in the body. They send and receive chemical signals, allowing us to respond to stimuli (meaning you can see to read these words). They transport materials such as nutrients and waste products in and out of our cells, and both synthesise and repair DNA. In other words, they are pretty damn important.
Proteins are assembled from small sub-units called amino acids. These link together by chemical bonds into short chains called peptides, once again producing polymers (chains). Longer assemblies of peptides are known as polypeptides. Proteins are larger structures again, and consist of at least one, though more commonly several polypeptides joined together to form a macromolecule.
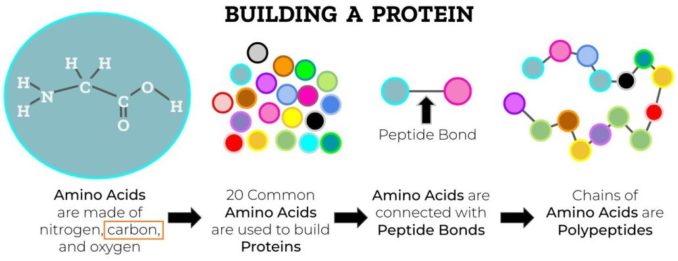
Adapted from SadiesBurrow, licensed under CC BY-SA 4.0
As the bonds form, the long chains twist, fold, and loop into unique, complex, and often quite beautiful, 3D structures. It is the structure that these macromolecules adopt which is crucial to their function.
To go further into this is well beyond the scope of this piece, so I will simply show you just a few of the amazing shapes (structural configurations) that proteins can take and give you some idea of just how massive a single biomolecule of protein can be.
To begin with, here’s a hormone you’ll probably be familiar with. Insulin, which regulates blood glucose levels. With a molecular formula of C257H383N65O77S6, it’s a pretty complex and sizeable molecule, comprising as it does, a chain of 51 amino acids. Its 3D structure was first determined in 1969 by the Nobel Prize-winning British chemist Dorothy Hodgkin at her Oxford laboratory, using the then fledgling technique of X-ray crystallography.
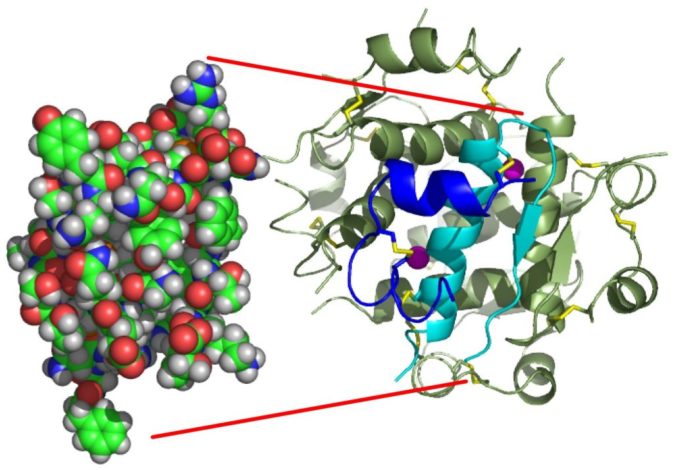
Isaac Yonemoto, licensed under CC BY 2.5
Looks pretty big, eh? Well, in molecular terms perhaps it is, but just take another look at insulin in the image below. This biomolecule can be found near the bottom—it’s that tiny green squiggle next to the elongated structure which represents an insect’s flight muscle protein. How massive in comparison, though, is the protein above it. That protein structure comprises a ‘virus’ which is thought to lead to an infection of the respiratory tract, a human adenovirus!
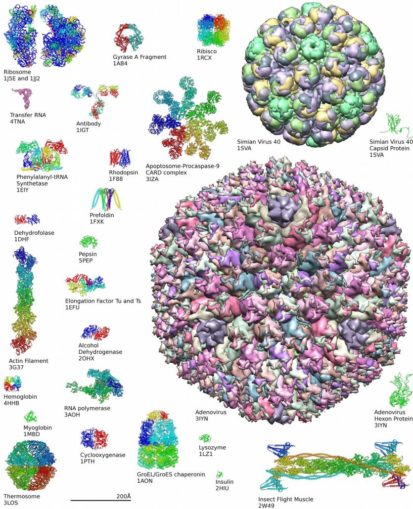
Axel Griewel, licensed under CC BY-SA 3.0
As we’ve already mentioned, the other main biomolecules are lipids and carbohydrates, and these both of these carbon-based molecules can and do interact with proteins. This makes them equally critical to how our bodies work, as well as linking to the causes of some of the body’s failings.
Protein biochemistry, and structural biochemistry, research into which I was privileged to observe at first hand over nearly a decade, is a vast and incredibly complex field. A single article wouldn’t even scratch the surface, so I’ll just reiterate that not a single aspect of this phenomenal ‘chemistry of life’ would be possible without the humblest of elements, our friend carbon.
Now I’d like to take you on a little dating excursion: one which is of particular interest to me. No, I haven’t signed up to Tinder, this relates to carbon-14 dating, otherwise known as radiocarbon dating.
This technique, probably the most widely used scientific dating method in archaeology, was developed in Chicago in the mid-1940s by American physicist Willard F. Libby. It allows us to determine the age, up to about 50,000 years old, of an artefact or object which is made from or contains organic (carbon-based) material of biological origin. The method, for which Libby was awarded the Nobel Prize in Chemistry, is only possible because carbon-14 (sometimes referred to as radiocarbon, 14C or C-14) is radioactive.
How does this work in practice? Well, we mentioned three naturally occurring isotopes of carbon earlier in this piece. The rarest of these, 14C, is created when a stray neutron (formed by cosmic rays hitting atoms in the upper atmosphere) bumps into an atom of nitrogen (which has seven protons, and seven neutrons).
This high-energy collision knocks a proton out of the nitrogen atom, replacing it with the stray neutron. This transforms it into a carbon-14 atom (a carbon atom with six protons, and eight neutrons), also creating a hydrogen atom (one proton, zero neutrons).
This slightly random process means that 14C atoms only exist in trace amounts. There aren’t many 14C atoms in comparison to 12C which makes up nearly 99% of all carbon atoms, nor the rarer 13C. However, since each isotope exhibits near identical chemical properties to those of the others, they all take part in chemical reactions, such as the creation of carbon dioxide, in the same way.
Plants absorb carbon dioxide by photosynthesis, and this is incorporated into all plant tissues. Animals and people eat plants, either directly or via a more complex food-chain, so also take up 14C. The ratio of normal carbon (12C) to 14C in the atmosphere and in all live organisms is virtually constant. However, as soon as an organism dies (whether a plant or an animal), it no longer takes in new carbon.
Now that 14C is no longer being constantly replenished in the material, it decays over time at a given rate, i.e. its half-life, 5730 ± 40 years. By measuring the ratio of 12C to 14C in a sample (a formerly living thing) and comparing it to the ratio in a living organism, it is possible to determine the age of the sample relatively accurately.
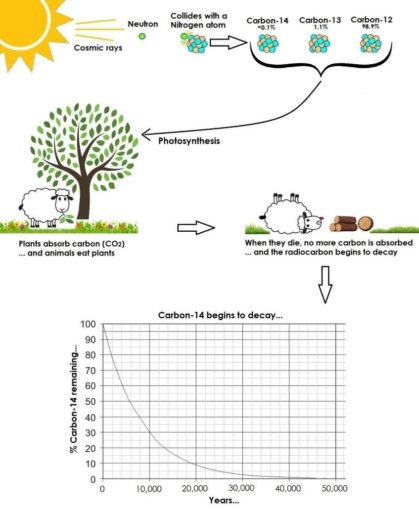
SharpieType301, 2023
You might have wondered why it’s only possible to determine the age of an artefact up to about 50,000 years old. Well, looking at the graph in the image above, you can see that after around 30,000 years old, only a small proportion (c. 3%) of the original 14C remains. As this percentage decreases further over time, and the graph continues to flatten, it becomes much more difficult to provide an accurate date.
Is it a perfect technique? Well, no, like so many things it’s a powerful tool, but has its limitations. Samples have to be wisely selected, to provide a good contextual relationship between the material to date and the period of interest. They must be meticulously collected and carefully handled to avoid contamination. The size of sample required, though usually only in the tens of milligrams, can pose a challenge to acquire in an archaeological context, where a given artefact may be quite small and extremely precious. All this said, it offers a glimpse into the past which we would not otherwise have. All this relies upon our element of interest here, carbon.
Whilst we are on the subject of archaeology, I couldn’t leave out the contribution carbon made to our ancestors’ use of metals. Whilst metals such as copper in their native form were in use much earlier, a huge step forward in technology came with the realisation that it was possible to extract some metals from their ores.
However, you cannot simply melt the metal out of an ore as the metal is oxidised (it’s part of a compound), so a chemical reaction is required to strip away the oxygen. This is where carbon comes in.
Where the metal in an ore is a less reactive element than carbon (for example, iron), carbon can, when heated strongly, displace the metal from its compounds by reacting with the other element (e.g. oxygen from the oxide). This is the basis of smelting.
e.g. iron(III) oxide + carbon → HEAT → iron + carbon monoxide
The first metal known to have been smelted was lead, with the oldest cast lead discovered (in the form of beads) dating to around 6,500 BC. These were excavated at Çatalhöyük, a Neolithic site on the Konya Plain in southern Anatolia. A Chalcolithic metal workshop dating to around 5,500 BC with a furnace and copper tools was excavated at Pločnik, in Serbia, with the oldest securely dated evidence of copper smelting at high temperature was found further north in Serbia, at Belovode. Tin is known to have been smelted by around 3,000 BC at Kestel near Göltepe, in the Taurus Mountains of south-central Turkey, as well as at sites in the Balkans.
It was the increased exploitation of tin which heralded the beginnings of the Bronze Age, around 3,000 BC. It was observed that deliberately including a second metal with copper (creating an alloy) made the resulting material more durable and harder—a significant step forward where bladed weaponry and armour was concerned. The addition of tin also lowered the melting temperature, leading to improvements in the ease of casting. This, in turn, led to an explosion in the use of bronze for a huge variety of artefacts. From ornamental and decorative pieces and statuary, to jewellery, ritual objects, bowls, and other vessels, to tools and nails, and of course weaponry and armour. Bronze was almost ubiquitous.
But technology rumbles ever onwards, and the new kid on the block would be iron. The Hittites of north-central Anatolia were the first to recognise its potential, and iron smelting was in use by around 1,500 to 1,200 BC. This practice soon spread, first through the Near East, then more widely, across the Mediterranean and into Southern Asia. One could say that increasing use of carbon in metalworking alone, was a driver in the progression of modern societies.
It, of course, doesn’t end here. The Middle Ages (late 5th to 15th centuries) saw the production of wrought iron (pig iron) from cast iron, using finery forges. What was used as the primary fuel? Yep, carbon, in the form of charcoal. The next development was steel, and the high-carbon crucible steels like Wootz steel and Damascus steel, with their characteristic ‘watered’ patterning, rightly famed their durability and ability to maintain a keen edge. For a good overview, see An introduction to Japanese knives from our very own Bertram Gilfoyle.
I’m aware that this article has turned into something of a marathon. There’s such a lot more that could be said about this incredible element, not least around the modern shenanigans about CO2 and climate change. However, I’ll leave it here, with one last thought about coal.
It’s generally regarded as a form of carbon, although it isn’t pure carbon. Coal also contains other elements that made up the original living source (e.g., nitrogen, hydrogen, sulphur, and oxygen). Over geological time (millions of years), those living things were converted by heat and pressure into coal.
The romantic poet Christina Rossetti (1830-94) reminds us, in what now seems quite a timely little poem given the steep rise in energy prices in recent months, of the value of coal. She points out that both diamond and coal (made of the same element, carbon), have great worth. It simply depends on how one looks at value.

Adapted from “The Rossettis: Dante Gabriel and Christina”, Public domain
© SharpieType301 2023



