In the previous article, we looked at the development of the Periodic Table (a.k.a. the Periodic Table of the Elements). The time has come to have a more in-depth look at the story behind some of the stars of the coloured squares, the elements themselves.
Taking a leaf out of Julie Andrews’ book, let’s start at the very beginning (a very good place to start) with Hydrogen.
Image from Modified from 2012rc, licensed under CC BY 3.0
This element was first noted, though not recognised as an element, in the early 1500s by the alchemist, physician (the two go together surprisingly often) and all-round rebel, Theophrastus Bombastus von Hohenheim. With quite a name to conjure with, this remarkable man, otherwise known as Paracelsus, like Mendeleev in the previous article, also has a station named for him, Paracelsus-Bad on the Berlin U-Bahn.
No slouch, Paracelsus, one of his hypotheses was that all natural substances have helpful characteristics, and harmful ones. He is quoted as saying:
“Alle Dinge sind Gift und nichts ist ohne Gift, allein die Dosis macht es, dass ein Ding kein Gift ist.”
This roughly translates as ‘all things are poisonous, but it is only the dose that determines whether it is poisonous’, which led to him becoming known as the ‘father of toxicology’.
Image from Jakob Suckale, licensed under CC BY-SA 3.0
This was actually a pretty bold statement at a time when illness was seen as an imbalance of Galen’s four humours: blood, phlegm, choler (yellow bile), and melancholy (black bile). To be healthy, a person’s humours needed to be perfectly in balance. In fact, Galen’s humours were closely linked with Aristotle’s classical elements as seen in the previous article, and the temperament of an individual. Moving away from this dogma was tantamount to heresy.

Image from Wellcome, licensed under CC BY 4.0
He was also a firm believer that, whilst not quite a panacea, beer had healing powers. It goes without saying that there’s been a brew named in his honour. Brewed by Stiegl, Austria’s most successful and biggest brewery, ‘Paracelsus Zwickl’ is a cloudy, organic, unfiltered, unpasteurised, gluten-free beer. I haven’t tried it, but if I ever see it, I shall.
In 1520 Paracelsus observed that when metals (e.g., iron, zinc, or tin) were dissolved in sulphuric acid, a gas was produced. Similar experiments were conducted over the next century or so, most notably in around 1650 by the Swiss physician Sir Théodore Turquet de Mayerne who remarked that the gas which evolved was flammable, so called it ‘inflammable’ air. This was followed up in 1700 by Nicolas Lemery, who realised this gas was explosive in air.
However, that’s where it stayed until hydrogen was ‘discovered’ (although isolated and its elemental nature formally recognised might be a better way to put it) in 1776 by the English natural philosopher, chemist and physicist Henry Cavendish. He observed that this flammable substance formed water on combustion, so called it ‘dephlogisticated’ or ‘inflammable’ air.
A man of substantial independent means (he inherited two considerable fortunes), Cavendish was known as ‘the richest of all the savants and the most knowledgeable of the rich’. It is ‘The Honourable Henry Cavendish’ after whom the University of Cambridge’s famous Cavendish Laboratory is named.

Wikimedia Commons, licensed under CC BY 4.0
Much of his work, though never published but evident from the copious notes he kept, suggests that he was streets ahead of other scientists who gained the credit for fundamental physical and chemical principles that Cavendish had already figured out. Although obviously brilliant, and hugely respected, he was a secretive, solitary, shy, and self-effacing individual. Indeed, it has been suggested that Asperger’s syndrome might be behind this personality trait of the great man.
The name hydrogen was actually coined in 1783 by the French chemist Antoine-Laurent Lavoisier, who we encountered with his wife, Marie-Anne, in the previous article. This rebranding came as part of his work to create a rational system of chemical nomenclature. He disproved Johann Joachim Becher’s theory of phlogiston (this being a substance that all flammable materials were believed to contain, and which was released when the material burned) and renamed this ‘inflammable air’ hydrogène, using Greek roots for ‘water’ (hydro) and ‘forming’ (genes).
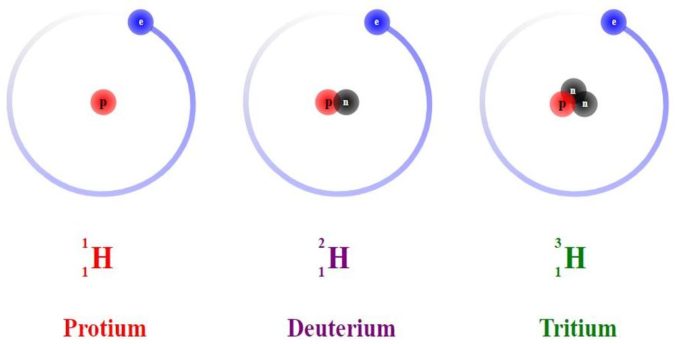
Hydrogen (symbol H) is a non-metal element. It exists as a gas in nature as a molecule made up from two hydrogen atoms, H2. It is colourless, has no odour nor taste, and is non-toxic, although it is highly combustible. Hydrogen is the lightest element on the periodic table, and the simplest. Having the atomic number 1, it has just a single electron and one proton in its nucleus. It is the only element without a neutron, at least in its most common form. Incidentally, in this, its most common form, hydrogen is sometimes called protium (symbol 1H), but there are other, rarer, forms of hydrogen (these are known as isotopes).
As far as I am aware, every chemical element has at least one, often more isotopes. An isotope is where two (or more) forms of an element exist, but only differ because their atoms have the same number of protons in the nucleus, but a different number of neutrons. Atoms of each isotope will have the same atomic number but a different atomic weight (also called mass number). Generally speaking, when compared with the number of protons, the more neutrons in the nucleus the less stable that nucleus (and, thus, that isotope) will be.
The most stable of hydrogen’s isotopes is deuterium, a.k.a. heavy hydrogen (symbol 2H) which was first detected in 1931. 2H is found at a ratio of 1:6420 to 1H and has twice the mass of normal hydrogen as its nucleus does contain a neutron. This has a variety of uses, both scientifically and commercially, including in NMR, in pharmaceuticals, and in both nuclear reactors and weaponry.
Dirk Hünniger, licensed under CC BY-SA 3.0
Tritium (symbol 3H), with two neutrons in its nucleus is the next most stable, although this is a radioactive isotope, and has a half-life of just over twelve years. Tritium was used in the past as a radioactive tracer in chemical and biological experiments and has found some use in self-powered lighting. There are additional, more unstable isotopes, 4H, 5H, 6H and 7H, each having an additional neutron in the nucleus.
Hydrogen (in its 1H form) is the most abundant element on earth. It is mainly found in combination with other elements (in compounds) and is found in huge quantities as part of the plentiful supply of water on our ‘blue planet’. This water is in oceans, lakes, rivers, and underground aquifers, frozen in icecaps and glaciers, and in the atmosphere.
Perhaps even more importantly, hydrogen is a fundamental part of the organic and other compounds (like water) which make up the bodies (including vegetable and animal tissue) of every living thing on Earth, including ourselves. Approaching ten percent of an organisms’ weight is hydrogen (primarily in proteins, fats, and water). Though it’s essential to all life, hydrogen’s role is not a directly active one.
It tightly bonds to other elements to form the building blocks of life—biomolecules such as nucleic acids (RNA and DNA), proteins, lipids, and carbohydrates are reliant on hydrogen bonds for their structure. It is also central to much smaller functional molecules such as metabolites, messenger molecules (e.g., hormones, pheromones, and neurotransmitters), and energy carriers such as ATP. It is produced by algae and some bacteria and, along with methane, occurs naturally in farts.

Purple Slog, licensed under CC BY 2.0
It isn’t only of vital importance on Earth. Hydrogen is also the most abundant chemical substance in the universe, accounting for around 73-75 % of all matter. Our Sun, indeed, most stars are largely hydrogen (in the form of plasma). Some planets too, e.g., gas giants Saturn, Jupiter, Uranus, and Neptune, are predominantly hydrogen. Even in the void, the supposedly empty darkness of interstellar space, a few atoms of hydrogen can be found.
Hydrogen is generally considered to be one of the primordial elements, originating following the Big Bang (an event which took place some fifteen billion years ago that I singularly fail to get my head around). It is believed to have formed as the rapidly expanding universe cooled sufficiently to allow subatomic particles to form then, soon afterwards, for the resulting electrons and nuclei to combine into atoms.. and not simply of hydrogen. A decent explanation of this phenomenon was given by our friend Rat Catcher in The Sun – page 2 unfortunately….
This means that hydrogen predates all other elements, even helium which was formed at around the same time. Pretty amazing stuff, eh?
As we have seen, it is found in nature as a gas. However, Scottish chemist and physicist James Dewar (he of the vacuum, or Thermos flask) devoted a good part of his research into the properties exhibited by matter at low temperatures approaching absolute zero (−273.15 °C, or 0 K [kelvin]). He built a large ‘regenerative cooling’ refrigerating machine which allowed hydrogen to be collected as a liquid for the first time (in 1898), and in solid form the following year (1899), in fact achieving a low-temperature record for the time, of −252 °C (21.1 K).
Hydrogen has the lowest density of all gases. Being significantly lighter than air, it can be used to provide lift. Taking advantage of this, it was used in the first unmanned gas balloon flight, conducted in Paris in 1783 by Jacques Charles and Les Frères Robert. This came in the very same year that hydrogen acquired its name. As a gas balloon has great buoyancy, it can be made smaller than a hot air balloon and, since it does not need to be topped up like hot air, will also have a considerable range. This made hydrogen filled balloons, airships, and dirigibles a good prospect, commercially.
That said, their development had quite a chequered history, not least due to the flammable nature of the gas. Nevertheless, by 1919, the Beardmore HMA R34 made the first Atlantic crossing by hydrogen airship, and in 1929, the LZ 127 Graf Zeppelin made the first and only circumnavigation of the world by airship, a flight of 33,234 km. Significant achievements indeed, and by the mid-1930s, passenger carrying airships designed and built by the Zeppelin company operated a service across the Atlantic, completing successful flights between Germany and Brazil in 1936.
Unfortunately, one of these airships, the LZ 129 Hindenburg, burst into flames just prior to mooring at a mast at Lakehurst, New Jersey on 6th May 1937. It rapidly became completely engulfed by fire, dramatically crashing to the ground with significant loss of life.

Recuerdos de Pandora, licensed under CC BY-SA 2.0
Because newsreel footage was seen around the world, this well documented event destroyed public and industry faith in hydrogen filled airships, effectively ending the promising days of international commercial passenger flights.
Perhaps it’s too easy to forget just how risky using this gas can be for manned flights, or maybe with time, hubris kicks into play. Whilst not the direct cause, a leak in the liquid hydrogen (LH2) tank of NASA’s Space Shuttle ‘Challenger’ certainly contributed to the horrific explosion which led to the death of its seven crew members, just over a minute after launch in January 1986.
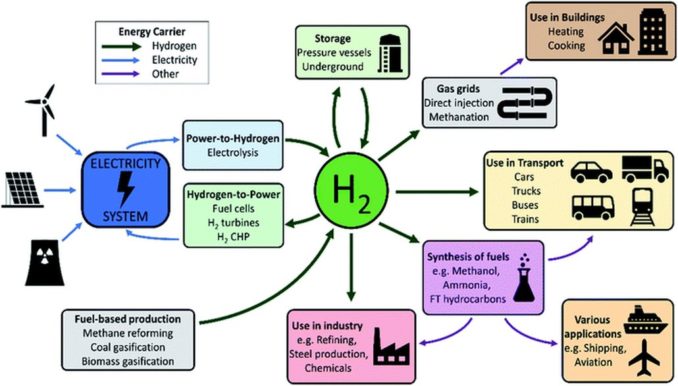
But hydrogen, despite this bad press, is still used in weather balloons and as a fuel. It also has many other practical uses. In science, it is invaluable in many analytical techniques e.g., gas chromatography, atomic absorption spectroscopy, and nuclear magnetic resonance. It’s crucial in cryogenic research, including superconductivity experiments.
Since it can be used as a coolant (e.g., in large electrical generators), and as a highly reactive gas, it is also used in many different sectors of industry. This includes the production other chemicals, for example methanol, which is used as a fuel, ammonia for fertilisers (this is, in fact, its principal industrial application), chiefly for the fertilizer market and domestic cleaning products, and hydrogen peroxide, which is widely used in medicinal products, as a bleaching agent, and to grow a wide range of plants hydroponically.
It has applications in the production of electronics and semi-conductors, and in the manufacture of textile fibres (e.g., nylon, and polyurethane). It is used in pharmaceuticals and foodstuffs (e.g., producing sweeteners like mannitol, or to hydrogenate unsaturated fats), in metallurgy (as a reducing agent to extract metal from ores) and in commercial glassmaking to manufacture plate glass. Its high reactivity means it is used in welding both as a shielding gas, and to produce an extremely hot torch. Reaching temperatures of c. 4000°C, this is capable of melting steel.
The Royal Society of Chemistry, licensed under CC BY 3.0
Another extensive user of hydrogen is the petrochemical industry. From hydrocracking to break down the long-chain hydrocarbons found in heavier refinery products, to the removal of sulphur in crude oils in refining petrol, jet fuel, kerosene, diesel, and fuel oils, it has found widespread uses.
But as you’ll see from the image above, to ‘go green’ and meet zero-carbon targets, the spotlight has returned to using hydrogen as a fuel once again. A massive amount of research is being devoted into vehicles of all types which could use hydrogen as a fuel to provide their motive power. Alongside this, a great deal of criticism is also being levelled at this ‘great step forward’. A useful overview can be found HERE, so I shan’t go into any further detail.
Not that this is a new idea, by any stretch. Igor Sikorsky proposed using liquid hydrogen as a fuel in 1938 and, nearly a hundred and fifty years ago, back in 1874, Jules Verne wrote of a world where “water will one day be employed as fuel, that hydrogen and oxygen of which it is constituted will be used”

Recuerdos de Pandora, licensed under CC BY-SA 2.0
Finally, we’ve already seen hydrogen’s potential for destruction, and that’s when it explodes accidentally. Mankind being the sane and sensible occupiers of our planet that we are, of course minds would be turned to the ‘what if’ of using its power deliberately. Yep, I’m talking about the H-bomb, and couldn’t possibly finish looking at this element without a quick gallop through this fearsome and rather questionable technology.
The first full-scale test of a thermonuclear device, or hydrogen bomb (H bomb) took place on November 1, 1952, codenamed ‘Ivy Mike’. These thermonuclear or fusion weapons are sophisticated second-generation nuclear weapons with enormously greater destructive power than first-generation nuclear bombs. They can also be built more compactly with a lower mass, so are more easily transported. Win, win, eh?
To be honest, the first-generation devices were pretty damned effective, as was demonstrated by the Americans in WWII, deployed against the Japanese cities of Hiroshima and Nagasaki. The first of these, ‘Little Boy’, exploded at Hiroshima with the power of approximately 15 kilotons of TNT. Its explosive power came from the nuclear fission of uranium-235.
Then came ‘Fat Man’, detonated over Nagasaki. This device yielded the power of approximately 21 kilotons of TNT and was an implosion-type design with a solid plutonium core.
Both of them pale in comparison with the most powerful nuclear weapon ever created, the second-generation ‘Tsar Bomba’ tested on 30 October 1961 by the Soviet Union. This behemoth yielded something in the region of 50–58 megatons, not kilotons, of TNT.
This is over 1,570 times more powerful than the combination of the two bombs dropped on Hiroshima and Nagasaki. The ‘Tsar Bomba’ fireball alone was 5 miles wide, with the resulting mushroom cloud some 25 miles wide at the base, and nearly 60 miles wide at the top. The radius of what was categorised as the zone of ‘severe damage’ extended for some 150 miles around the point of detonation. The cloud reached around ten times higher than that of ‘Little Boy’, to an altitude of around 200,000 feet, almost into the Earth’s mesosphere. That’s nearly six times higher than a commercial airliner typically flies.
Perhaps scarier still, is that this test made use of a bomb which was significantly smaller than had been originally intended. The initial design for the bomb would have had an anticipated yield in excess of 100 megatons!
We’ve recently been warned that we are currently at the ‘highest ever’ risk of nuclear Armageddon, and the hands of the scaremongering ‘Doomsday Clock’ have been set at 90 seconds to midnight. I have recently dug out my old copy of Protect and Survive (OK, it’s a facsimile, but who’s counting), but if the superpowers were to go head-to-head with these beasts, I suspect it’d do me no damn good at all.

Image from SharpieType301, 2023
If you’d care to spend a bit of time scaring yourselves witless, Auntie Beeb produced a documentary in 2017 called ‘The Bomb’. Our friend Rat Catcher wrote a nice piece, ‘How I learned to despise the left and fear the bomb‘, in the same year on the psychological impact that ‘the bomb’ on all of us of a certain generation. Jim Walshe gave us his ‘Postcard from Hiroshima’ to contemplate, and I provided a little of the long history of nuclear weapons testing in ‘Operation Teapot’, though this was series of tests was conducted with the nominally ‘less scary’, more ‘conventional’ first-generation bombs.
On that cheery note, I’ll finish a look at hydrogen, the first of our elements. The next article will explore another of the more commonplace elements, though I’ve not yet decided which, nor when I’ll get round to writing it.
In the meantime, I think Tom Lehrer expresses my thoughts better than I ever could, so let’s sing along…
Tom Lehrer – We Will All Go Together When We Go
When you attend a funeral
It is sad to think that sooner o’
Later those you love will do the same for you
And you may have thought it tragic
Not to mention other adjec-
Tives, to think of all the weeping they will do
(But don’t you worry.)
No more ashes, no more sackcloth
And an armband made of black cloth
Will some day never more adorn a sleeve
For if the bomb that drops on you
Gets your friends and neighbors too
There’ll be nobody left behind to grieve
And we will all go together when we go
What a comforting fact that is to know
Universal bereavement
An inspiring achievement
Yes, we all will go together when we go
We will all go together when we go
All suffuse with an incandescent glow
No one will have the endurance
To collect on his insurance
Lloyd’s of London will be loaded when they go
Oh we will all fry together when we fry
We’ll be french fried potatoes by and by
There will be no more misery
When the world is our rotisserie
Yes, we will all fry together when we fry
Down by the old maelstrom
There’ll be a storm before the calm
And we will all bake together when we bake
There’ll be nobody present at the wake
With complete participation
In that grand incineration
Nearly three billion hunks of well-done steak
Oh we will all char together when we char
And let there be no moaning of the bar
Just sing out a Te Deum
When you see that I.C.B.M.
And the party will be “come-as-you-are.“
Oh we will all burn together when we burn
There’ll be no need to stand and wait your turn
When it’s time for the fallout
And Saint Peter calls us all out
We’ll just drop our agendas and adjourn
You will all go directly to your respective Valhallas
Go directly, do not pass Go, do not collect two hundred dolla’s
And we will all go together when we go
Ev’ry Hottenhot an’ ev’ry Eskimo
When the air becomes uranious
And we will all go simultaneous
Yes we all will go together
When we all go together
Yes, we all will go together when we go
© SharpieType301 2023



