By now, you should have (happily I hope) settled into our explorations of a day in the life of the elements. So far, we’ve investigated Hydrogen, Carbon, Mercury, Bromine, and Oxygen, taken a canter through some basic chemistry, and had a look at how the Periodic Table (a.k.a. the Periodic Table of the Elements) developed.
This time, with steely resolve, we’re embarking on our seventh voyage of discovery. Unbending in our pursuit of interesting snippets, our element this time is the one our periodic table below chose to highlight, so that it could explain all of those detailed little annotations.
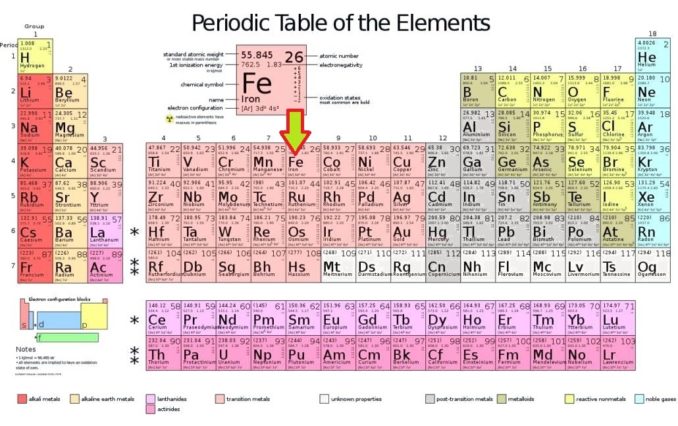
It’s time to meet the most common element on Earth (by mass), Iron, a metal which could be said to be the most useful of all of the elements, something hinted at by Stanley Holloway in his 1960 hit Any Old Iron.
2012rc, licensed under CC BY 3.0
With iron, we are looking at the first of the elements in Group 8. This group doesn’t have a fancy ‘trivial’ name, which I feel is a bit of a shame, but it is part of the lovely salmon pink transition metals section of the table, sometimes also known as the d-block elements. All are metallic, exactly as you’d imagine, being found amongst the ‘transition metals’. As well as iron (Fe), the group includes ruthenium (Ru), osmium (Os) and the radioactive hassium (Hs).
As to what a transition metal actually is? Ah well, she says, scratching her head… it’s kind of complicated. More or less, they are elements which, because of the way in which their electrons are arranged, can take part in chemical bonding using electrons, not just from the outermost shell (as is usual) but from the outer two electron shells. From here I’m going to take the coward’s way out and not attempt to provide a better explanation (mostly because I’m not convinced there is a ‘simple’ one). If you’d like to know more, there’s some good information HERE.
In older, pre-IUPAC* periodic tables, which used an earlier naming protocol, the group 8 elements were actually combined with their neighbouring elements in groups 9 and 10. Together, these elements, which exhibit significant chemical similarities, were termed Group VIII B. (*IUPAC is the International Union of Pure and Applied Chemistry. It is this body who are responsible for assessing whether a new element has been ‘discovered’ and deciding upon a name).
The Group 8 elements are a dense bunch, in fact, the element which is the densest to occur naturally is iron’s cousin osmium. Interestingly, according to NASA’s Jet Propulsion Laboratory, iron is the heaviest element that can be formed in the cores of stars. Elements heavier than iron can only be created when a supernova occurs (when a high mass star, like our Sun, explodes).
Our name for ‘iron’ comes from the Anglo-Saxon word ‘iren’, which some etymologists associate with ‘strong metal’, although others think there may be a link to ‘holy metal’. There are similar words in Old Saxon and Old Norse ‘isarn’, and all of these words may derive from an earlier Celtic word ‘isarnon’. However, the symbol we give this element is Fe, which comes from the Latin word for this metal, ‘ferrum’.
Iron as an element wasn’t ‘discovered’ as such, so this time there are no jolly tales of competing chemists in different countries champing at the bit to ensure their remarkable breakthrough is the one first recognised. Instead, iron just kind of ‘is’, having been known about for millennia. In fact, iron is an element which was recognised as one of the ‘seven metals of antiquity’.

SharpieType301, 2023, images from Chemical Elements, licensed under CC BY 3.0
Iron, like many of the elements we’ll look at, has a number of different isotopes. Of these, four (54Fe, 56Fe, 57Fe, and 58Fe) are stable. The one we are most familiar with, 56Fe, comprises just under 92% of the iron found on Earth. Another stable isotope, 57Fe, has an application in Mössbauer spectroscopy, an adaptable quantitative technique used to determine the valence (or oxidation) state of iron in sample materials. It can provide valuable information about the structural, chemical, and magnetic properties of the sample, and finds applications in the study of geological materials, ceramics, glass, and toxic waste. Mössbauer spectroscopy is also used in metallobiochemistry (bioinorganic chemistry), to study proteins and enzymes which contain iron.
Currently, iron’s other stable isotopes don’t have any notable applications. However, in addition to its stable isotopes, iron has a further twenty-four radioactive isotopes. Some of the longer-lived ones (those with a relatively long half-life) have found considerable use as tracers in medical and biological studies. Both 55Fe, and 59Fe have been used as a means to determine how iron (whether ingested or transfused) metabolises in the body.
Iron also exhibits different allotropes, that is different crystal structures, depending on the temperature and pressure at which the iron is examined. These are α-Fe (alpha iron) which most room temperature iron will be, γ-Fe (gamma iron) which forms as iron is heated above 912 °C, and δ-Fe (delta iron) which is formed at above 1,394 °C. These changes in structure are important to the physical properties of the metal, thus the uses which can be made of it.
Elemental iron is a solid at room temperature, a glossy silver-grey metal (see alpha iron above). It doesn’t stay that way for long though, as it rapidly oxidises (rusts, in common parlance).
iron + water + oxygen → hydrated iron(III) oxide
The metal reacts with water and oxygen in the air to form hydrated iron(III) oxide, the red-brown, flaky material we’re all too familiar with.

Tony Harrison, licensed under CC BY-SA 2.0
This propensity to oxidise means native iron is found only extremely rarely. Meteoric iron, in reality an iron-nickel alloy, is the exception here, and evidence shows that this was the earliest iron used. Before technology was developed to extract metallic iron from its ores, this was the only usable source of the metal.
It was seen as a mystical material, a gift from the gods, so was highly regarded as a rare and exotic material. The ancient Egyptians recorded it in hieroglyphic documents, calling it ‘bia-n-pet’, which roughly translates as ‘iron of heaven’ or ‘iron from the sky’.
The oldest (yet) discovered evidence of the metal’s use comes from nine worked iron beads, dated to c. 3,300 BC. These were excavated by the British School of Archaeology in Egypt team led by Gerald Wainwright in 1911, at a pre-dynastic cemetery site near el-Gerzeh in Egypt. These small beads, of rolled and hammered iron, predate the accepted start of the Egyptian Iron Age (c.1,200-1,000 BC) by more than 2500 years.
Obviously prized, the Gerzeh beads had originally been strung onto a necklace, interspersed with other valuable materials, such as gold and carnelian. Meteoric iron use has been identified from other high-status sites, including a number of objects found in the tomb of Tutankhamen, dating to c. 1,327 BC.
However, as it becomes more commonly found in the archaeological record, later iron objects are more likely made of iron extracted from ores. It has been said that the advances heralded by smelting iron ore to produce usable iron advanced civilisation to a similar degree to the domestication of animals.
Many forms of iron ore exist. Some may be silicates, sulphides, or carbonates, but the majority of usable iron ores are oxides. The primary oxide ores are haematite (Fe2O3), and magnetite (Fe3O4), and these two form the basis of ironmaking. They are iron-rich, typically up to c.60% iron content, and relatively easily processed. These ores are used as feedstock in furnaces where the ore is smelted to extract the valuable metal.
Originally, this extraction will have taken place in a simple bowl furnace or bloomery, and there’s a lovely video (in true understated British style) from the Wealden Iron Research Group showing how a bloomery operates. Astonishingly, in this way it is thought that iron may have been smelted in Central Anatolia as far back as 3,000 BC.
However, it took until around 1,500 BC or later for smelted iron objects to appear in Anatolia, Mesopotamia, and Egypt more regularly, and gradually replace bronze as the metal of choice for tools and weaponry. Around this time, smelting iron began in earnest, spreading across the region, around the Mediterranean, and more widely, thus initiating the period we now know as the ‘Iron Age’ at differing times in different areas of the world.
For many years it was thought that the ancient Indo-European Hittite civilisation had been the first to recognise the value of iron as a practical, utilitarian metal. The Hittites certainly made mention of iron in around two hundred of their documents, written in a variation of cuneiform, that have yet been translated.
However, archaeological evidence shows that the Hittites were not, as originally thought, the first large-scale iron producers. Nevertheless, adopting iron tools, weaponry, and armour which was not only superior to bronze but, being cheaper to produce, permitted them to arm of a large peasant infantry. This, and the development of fast war chariots, allowed them to develop as a dominant power in the Middle East, rivalling ancient Egypt.
There is evidence of air being introduced during the smelting process, using animal skin bellows and a nozzle (a tuyère). However, sometime later, it was realised that by forcing air into the furnace in a much more controllable manner, the ironmaking process could be made more efficient. The first ‘blast’ furnaces (dating to the 1st century AD) were in China, with water-wheel driven bellows providing a constant flow of air.
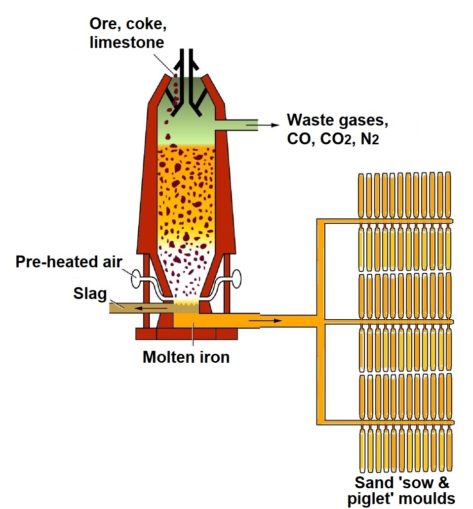
In a typical blast furnace, the iron-bearing ores, the fuel (usually coke, though originally charcoal), and a flux (a chemical cleaning agent, usually limestone) are fed in at the top of the furnace in a continual stream. Hot blasts of pre-heated air (hence ‘blast’ furnace) are blown into the bottom section of the furnace, rising through this mixture.
SharpieType301, 2023, adapted from philschatz, licensed under CC BY-SA 4.0
As the air and feed mingles, and the solid materials fall under gravity, chemical reactions happen throughout the mix, at temperatures of up to 1,200 °C. The reacting solids accumulate at the bottom of the furnace, as the molten iron forms together with the slag (a by-product). Both of these are run off, separately, from the base of the furnace.
This first smelt produces pig iron which, in its own right, has very limited uses. This is because it has a high carbon content (c. 3.8-4.5%) which makes it brittle. It also contains impurities like silicon, phosphorus, manganese, and sulphur. However, pig iron is an important step in the production of both wrought and cast iron, and steel.
Although, these days, re-usable heat-resistant moulds are often used, pig iron was once cast into thin ingots in sand moulds, pressed into the ground. The blast furnace is ‘tapped’ at the bottom, to release the molten iron. With sand-casting, this runs off down a central channel into a series of branching, interconnected runnels, then through narrower grooves into moulds set at right angles to the channel which, again, have been pressed into the sand.

SharpieType301, 2016
The pattern made by the moulds and furrows looks a little like a sow (the main channel) suckling her piglets (the rows of individual moulds). This resemblance gives the name ‘pig’ iron to the ingots. Once cooled, the moulded ‘piglets’ (ingots) easily break away from the stem of iron in the runnels. An example of these can be seen above, cast at the ironworks at Blaenavon in South Wales (well worth a visit if you find yourself nearby).
Before we go much further, it might be worth a quick look at the various types of ‘iron’ and ‘steel’ and what makes them different.

SharpieType301, 2023, images from Wikimedia
As a little aside, one which I only became aware of by not reading comments on an earlier article, there is a connection between iron and one of our favourite breweries. The village of Hook Norton sits in an area known as the ‘Redlands’, where ironstone (an iron-rich, and fossil-rich, sandstone) dominates. Indeed, the brewery (along with the nearby watering hole ‘The Gate Hangs High’) is constructed from this rich reddish stone. The ironstone stone was quarried from late 19th century through the first half of the 20th century (until just after the end of WWII), to supply the Brymbo Steelworks in Wrexham. Hooky have developed their Ironstone Lager in honour of this, and the bottle sports a nice line drawing of the ironstone-built brewery.
The development of different iron-based materials and the progress of technologies used in their production would make an article in its own right. Indeed, some aspects of this fascinating topic have been covered in Bertram Gilfoyle’s An introduction to Japanese knives, and I have a vague memory that other writers have included at least some details of different stages in the story of iron and steel. My hope is that another member of this august site might take the hint, harness their knowledge and experience, and do it justice. I, for one, would love to read it.
The uses of iron, and more particularly its alloys in the form of steel (since its properties can be closely tailored by tweaking its composition), are legion. The predominant use comes in construction—in buildings and other structural infrastructure, such as bridges, railways, etc. In this sector, it’s most often found reinforcing the structures, in the form of bars (e.g. rebar, used to reinforce concrete), beams, and sheets, but also in flooring, stairways, and other internal structures. It’s used aesthetically too, for sculptural details in modern architecture.
We know it from the cars and public transport, road, sea, and rail, we see (and often use) every day, but it is also used in bicycles, agricultural equipment, cranes, earthmoving equipment, storage tanks, and a vast number of tools (both hand and electrical). Stainless steels are found in settings where a hygienic or sterile environment is required. The low corrosion and ease of cleaning and maintenance make this material ideal for surgical instruments and implants.
It’s found in a large variety of consumer products, including small items such as razors (the best a man can get), cutlery, and food packaging. But it’s also common in other ‘essentials’, the larger domestic products like washing machines and dryers, ovens, microwaves, dishwashers, and refrigerators. It’s found in wiring too, in fact, it’s unlikely that you’ll find any electrical equipment that doesn’t have at least some steel in its makeup. Iron, and its derivatives, really is the most versatile of materials.
On a more personal note, iron is an element with which I feel a deep connection. The threads of iron (and associated industries) run through both sides of my family but, most notably, I am the daughter of a steelworker.
Dad was one of many South Wales men who worked for industrial giants Guest, Keen and Nettlefolds at the East Moors Steelworks in Cardiff. Until it’s closure in 1978, GKN was a major employer in the region for the majority of the 20th century. A Valley’s lad, Dad joined GKN when he parted company with the RAF at the end of WWII and married my Mum.
For many years, the East Moors steelworks dominated the Cardiff skyline. It can be seen here in 1973 from across the Bay in Penarth. To the right in the picture lies an area called Splott (yes, that really is a place!), which is where my Mum lived. The lovebirds met when Dad was stationed at RAF Pengam Moors in neighbouring Tremorfa. He was billeted with Mum’s family in Splott, several of whom had jobs at the steelworks or at the Currans foundry.

Ben Salter, Public Domain
My Dad bore lifelong scars from his post-RAF job, having suffered an appalling accident at the works, falling part way into a slag pit when I was just a baby, in circumstances I never did fully uncover (I really don’t know how but, thankfully, he survived).
I have two ironclad memories of those years. The first is being allowed to climb on ‘Jessie’, a retired steam engine from East Moors which was installed in Splott Park, just down the road from my Nan’s. Happy times. The other was a family outing by motorbike & sidecar, being taken (probably while we were visiting my Nan, as it was close by) ‘to see where Dad worked’. As a child, it was thrilling to hear the roar from the East Moors furnaces, and see the sparks and rich, red glow from a huge ‘shed’ where steel was being made. The experience certainly brought to mind Sunday School tales of Shadrach, Meshach, and Abednego, and ‘the firey furnace’.
Time for a few more fascinating ferrous facts. Whilst we are thinking about the Old Testament book of Daniel, did you know that the reference to weakness we are probably all aware of, having ‘feet of clay’, comes from here but also has an intimate connection to iron? In fact, in interpreting King Nebuchadnezzar’s worrying dream, Daniel (in Daniel, 2:42) describes the feet as being a mixture “And as the toes of the feet were part of iron, and part of clay, so the kingdom shall be partly strong, and partly broken.”
Indeed, iron features as an important and symbolic substance in the Bible, occurring in well over a hundred verses. The first blacksmith, “the forger of all implements of bronze and iron“, and the ‘father of smithcraft’ comes to us from Genesis. The Bible tells us of a descendant of the ‘ungodly’ line of Cain, a man described by the Judeo-Roman historian Flavius Josephus as one who “exceeded all men in strength”. Seven generations on from Adam and Eve, the son of Lamech and Zillah, this was Tubal Cain, an important figure in Freemasonry.
Symbolism and iron have close ties. The planet we know today as the ‘Red Planet’ (Mars) gets its nickname from its distinctive rusty-red colouration. This planet, like our own, has a largely iron core. The colour we see comes from a preponderance of iron(III) oxide (ferric oxide, Fe2O3) in the covering of loose sand, dust and rock deposits which makes up its surface.
Mars was so named by the Romans after their god of war, who was also a guardian of agriculture. In fact, the place we know as Rome wouldn’t exist without him as, in the legend of the founding of that great city in the 8th century BC, he sired the twins Romulus (for whom it was named) and Remus.
Perhaps The Romans selecting the name Mars should not be too great a surprise. The planet had been known by other warlike names, way back to the Sumerians who, from c. 4,500 BC, had called the planet Nergal, after their ‘god of inflicted death’. It is quite conceivable that the blood red colour the planet displays has long been symbolic of conflict and brutal death.
The Romans assigned the symbol of their god (a stylised shield and spear) to the planet, both astrologically and astronomically (the disciplines didn’t separate until around the 17th century). However, natural philosophy also extends to topics less celestial, with one branch being alchemy. We’ve already encountered the ‘seven metals of antiquity’ (not least in a previous article on mercury) and the symbols alchemists used for these metals.

SharpieType301, 2023
What I might not have mentioned was that each of these metals was associated with one of the seven planets visible to the naked eye. Our element iron was associated with the planet Mars, so shares its symbol. The curious thing, to my mind, is that the part iron plays in this planet’s colouration was most certainly not something that was nor could have been known in antiquity.
Perhaps equally thought-provoking is that, in 1751, the Swedish botanist Carl Linnaeus chose to use this self-same symbol to denote a biologically male organism (to convey female, he used the ideogram for Venus). Examining the glyph with this in mind, I’m not even going to speculate as to what might have given him this idea.
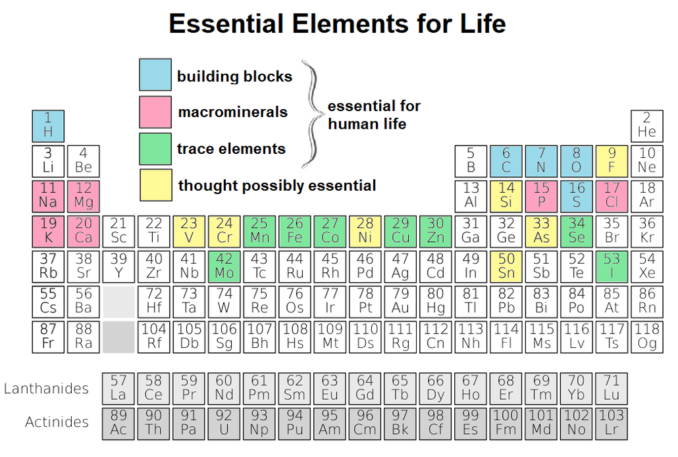
OK, with Linnaeus, we seem to have moved towards the world of biology. We’ve already seen from previous articles that oxygen and carbon are essential elements to living organisms.
Even though it isn’t included in the eleven elements (shown in pink and blue below) which are considered fundamental to life, iron too plays a very important role. It is actually still, albeit in trace amounts, essential for life. Indeed, iron is foremost amongst the trace elements which keep us, and almost all life forms, going.
A lack of iron in an organism will result in abnormal biological function, a complete absence, death.
SharpieType301, 2023, adapted from Cepheus, public domain
It isn’t just more complex life forms for which iron is a necessary element. Phytoplankton are one of the organisms (together with algae) which form the base of both marine and freshwater food webs. They obtain their energy through photosynthesis using carbon dioxide from the atmosphere (they are the watery equivalent of land-based plants), in the process producing somewhere in the region of half of the planet’s oxygen. Iron is a much-needed micronutrient for them to grow.
Thankfully, soluble iron compounds find their way into the oceans via airborne dusts, from sediments carried by rivers and melting glaciers, by volcanoes and from plumes of iron-rich water emanating from deepwater hydrothermal vents.
Being an essential nutrient, the presence of iron likely had an effect on the development of complex life forms, acting as a driver for evolution. Iron’s importance for humans cannot be overestimated, a fact which is all the more amazing when you consider that the adult human body contains, on average, only about four grams of this element. That’s roughly 0.005% of our body weight. OK, where is this bounty found and why is it needed?
Well, most living things, including ourselves, have within our cells an incredibly complex, globular protein called ferritin. This protein essentially acts as the body’s storehouse for the iron it requires, acting like a nanocage incarcerating up to 4500 iron(III) ions (charged atoms of iron in a soluble form) inside the protein shell. These it doles out, as required, when the body needs to adjust its iron levels (e.g., if more red blood cells need to be produced), since there’s a delicate balance between too much and not enough.
Ferritin isn’t the only heme-containing (iron-based) metalloprotein of importance. Some 70% of the total iron of the human body is present as haemoglobin, and c.3% is in the muscles as myoglobin.
Haemoglobin is the protein in red blood cells which not only gives them their red colour but acts as an oxygen transport system (you can imagine red blood cells as a whole cavalcade of bright red London double-deckers). It carries oxygen from the lungs (or gills, in non-mammalian vertebrates) through the bloodstream to other parts of the body. Then, once in the tiny capillaries of the tissues, it then releases that oxygen to other cells for use in aerobic respiration. This drives the chemical reactions which release energy from glucose, powering the organism’s metabolism.
Myoglobin, on the other hand, is located in muscle cells and specialises in providing the large quantities of oxygen needed by the muscles (including the skeletal and heart muscles). It is rarely detected in the blood unless it has been released by damage to the muscles. This makes it useful as an indicator of a heart attack in patients experiencing chest pain.
Interestingly, the first ever 3D structure of a protein to be determined using X-ray crystallography was myoglobin. This work was carried out in 1959 by John Kendrew and Max Perutz in the Cavendish Laboratory in Cambridge. Goodness, the world has moved on from those stylish and innovative days.

MRC Laboratory of Molecular Biology, licensed under CC BY 2.5
Ensuring the human body has the right amount of iron is critical to good health. An inherited disorder, haemochromatosis (most prevalent in those with Celtic ancestry), can cause a build-up of iron in the body. The body has no mechanism for removing this, so to rebalance the amount of iron may require regular portions of blood to be removed to lower the levels in the body.
Conversely, and more commonly, insufficient iron can cause problems. Although our bodies can and do recycle iron, we tend to lose a little iron every day and this needs to be replaced. If it isn’t, this lack can lead to iron deficiency anaemia. This is often picked up when an individual complains of fatigue, brain fog, feeling cold all the time, hair loss, shortness of breath, or heart palpitations. It can be confirmed by a simple blood test and remedied by eating iron-rich foods or taking iron supplements. Foods rich in iron include, amongst other things meats (particularly liver and other organ meat) but also beef and poultry, all kinds of seafood, nuts and pulses, and some leafy green vegetables.
Right at the beginning of this piece, I mentioned that iron could be said to be the most useful of all of the elements. Well, many seem to have held a similar opinion.
This can be seen in a few brief lines from a poem written in 1910by Rudyard Kipling (someone who was and remains a firm favourite of mine). Some of his poetry, in this case ‘Cold Iron’, was set to music by one of the finest traditional English folk singers you’ve probably never heard of. Not as crazy as it might sound—Kipling himself was known to hum or whistle as he wrote his poetry.

sammydavisdog, licensed under CC BY 2.0
Peter Bellamy set a number of Kipling poems to music (once Kipling’s daughter Elsie had passed away and no further objections stood in Bellamy’s path). Several of his recordings can be found on You Tube and they are well worth grabbing an Arran, sticking one finger in your ear, and having a listen.
I can’t leave our element without mentioning just a few quirky iron-related oddments, so shall close with these.
The first is a hat-tip to one of our Postal stalwarts, Judas was Paid. He has made many a gentle quip about his Iron Lung, so I had to include just a little about this extraordinary lifesaving device. Otherwise known as a negative pressure ventilator, this rather Heath Robinson-esque apparatus can help keep alive someone who has lost the ability to breathe unaided.
Our generation, or more commonly those who preceded us, are probably most familiar with it as the somewhat feared contraption into which school chums struck down with polio (poliomyelitis) might be consigned. Given the obvious rusting on this one, used at the Royal Victoria (or Netley) Hospital near Southampton, the name certainly seems apposite.

geni, licensed under CC BY-SA 4.0
You will also doubtless be familiar with the term ‘Iron Horse’, referring to a railway locomotive. It was one of those terms which somehow struck a chord and, perhaps even more strangely, has perpetuated. It comes from the early 1800s in Britain, when it was coined to compare the efficiency of the new-fangled steam locomotives with their transport predecessors, horse drawn vehicles Not only was this term used in Blighty but spread across the pond to the great Wild West, as railroads expanded across the untamed land in the mid-1800s.
When it comes to the term continuing in popular culture, you may be surprised to hear that there are two bands who have chosen to use this name. The first ‘Iron Horse’, a Scottish band, formed in the 1990s, played Celtic music like ‘The Steampacket’. The other bunch are an American Bluegrass band with a penchant for heavy metal songs made bluegrass. Their version of Van Halen’s ‘Ain’t Talkin’ ‘Bout Love’ (actually featuring David Lee Roth) gives you the general idea.
On the subject of heavy metal (which, of course, our element iron actually is), there’s also ‘Iron Maiden’, a band who hail from Mr Sharpie’s old stamping grounds in Leyton. If I’m honest, whilst they have their fans, and doubtless for good reason, they aren’t great favourites of mine. My memory of seeing them at the Rainbow in Finsbury Park sometime in the early 1980s is hazy at best. I can only assume they took their name from the rather hideous cabinet of spikes purported to be a mediæval instrument of torture. Interestingly, I don’t believe they used this ghastly image on any of their album covers, so who knows.
© SharpieType301 2023



