In this series, we’ve taken a wander through the marvels of the Periodic Table (a.k.a. the Periodic Table of the Elements) and become familiar with some fundamental chemistry principles. We have explored: Hydrogen, Carbon, Mercury, Bromine, Oxygen, Iron, Sodium, and, most recently, Copper.
Today, we’re going to take a look at a reactive non-metal, in fact, this is one of the most reactive of all of the elements. Despite its reputation, I think it is an absolutely beautiful element.
This episode will tell Sulphur’s story. Note, I shall use our good old English spelling for this element, not the one our Yankee cousins insist upon, and which drives me quietly batty whenever I see it.
Modified from 2012rc, licensed under CC BY 3.0
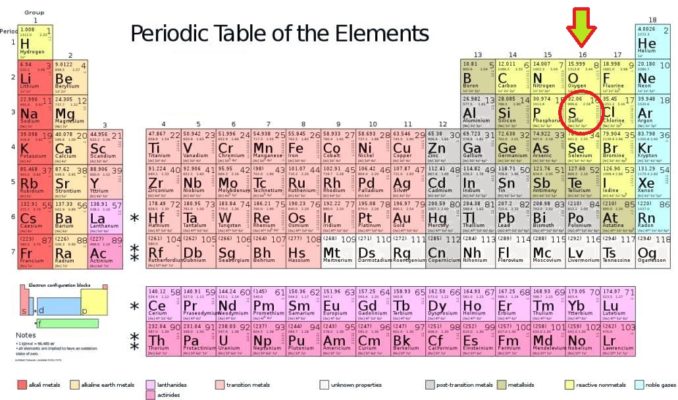
As you’ll see from the Periodic Table above (with American spelling – aaargh!), sulphur is a compatriot of oxygen, and lies in Group 16. This group is also known as the chalcogens, and we have already discussed the origins of this name, and some characteristics of other elements in Group 16 in a previous article.
Coincidentally, sulphur, which has been given the symbol ‘S’, is the sixteenth element on the Periodic Table. It is also the sixteenth most abundant element in the Earth’s crust. How cool is that?
Sulphur is a non-toxic solid, insoluble in water. It has poor thermal conductivity and acts as an excellent electrical insulator. Despite its traditional association with an unpleasant ‘rotten eggs’ smell, sulphur is actually both tasteless and odourless. In fact, the stinky substance is one of sulphur’s compounds, hydrogen sulphide (H2S), which was known by miners as ‘stinkdamp’. It isn’t alone, as a number of other sulphur compounds have a disagreeable smell.
Because it is such a reactive element, sulphur compounds abound, and it can react with nearly every other element (except the noble gases, a notoriously snooty lot who rarely give anyone the time of day!).
Why so reactive? Well, each sulphur atom (as for all the Group 16 elements) has six electrons in its outermost shell—this means it is hexavalent, lacking two electrons to fill it. This means that sulphur can act as either an oxidising agent or reducing agent, and have an oxidation state of -2, or -1 (by accepting electrons), alternatively +2, +4, or +6 (by losing electrons).
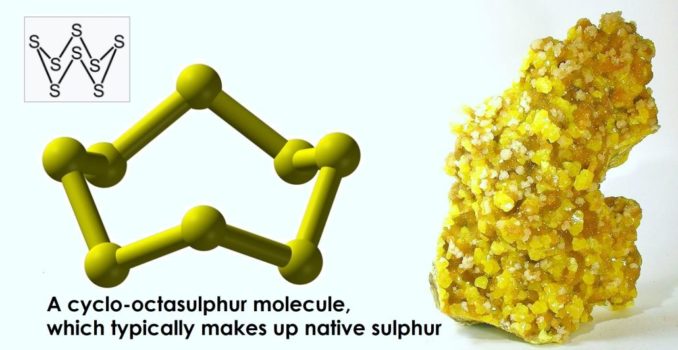
In relation to the human body, it is a very important element indeed, but more on that later. For now, let’s focus on the element itself, which occurs naturally in a pure form, as a relatively soft but brittle crystalline solid, often in volcanic areas. It is, perhaps, most recognisable for its intense daffodil yellow colouration, although when it burns, it does so with a bright blue flame (best observed in the dark) and melts into a deep blood red liquid. This ‘trinity’ of differences in colour seem pretty astonishing now but must have seemed truly magical to our early ancestors.
Adapted from Robert M. Lavinsky, licensed under CC BY-SA 3.0 and Wikimedia Commons, Public Domain
In elemental form, when found in nature as native sulphur, it usually exists as cyclo-octasulphur molecules, which exhibit a ring structure of eight linked atoms, S8. In shape, this looks something like a sort of ‘crown’, as you’ll see above. This is the form (allotrope, actually) most commonly encountered in ‘flowers of sulphur’ and ‘roll sulphur’, which you might remember from school.
Cyclo-octasulphur is also called rhombic sulphur or α-sulphur. The eight atoms in this molecule are linked by covalent bonds, where electrons are shared between atoms. It can form ring structures with different numbers of atoms, too. Albeit with a little chemical jiggery pokery in some cases, sulphur rings have also been identified which contain 6, 7, 9, 10, 11, 12, 13, 14, 15, 18, and 20 atoms.
Because of the different ways in which atoms of sulphur can bond, one to another, covalently (sharing electrons), sulphur is second only to carbon in the number of different allotropes which can form. There are three main allotropic forms: orthorhombic, monoclinic, and amorphous, of which the first is the most stable. At present, there are about thirty well-characterised allotropes of sulphur known, although several of these require high pressure to form. In fact, sulphur’s crystallography is rather complex, since it can adopt distinct crystal structures, depending on the conditions (it’s all a bit complicated, but there’s a nice overview on Wiki).
But what of its isotopes? Well, there are twenty-three isotopes of sulphur, of which four are stable: 32S (the most abundant), 33S (used in NMR spectroscopy), 34S, and 36S. Other isotopes are radioactive, and their half-lives are relatively short.
The distinctive isotopic composition of sulphur (as in, what percentage of specific isotopic forms are present within a raw yellow lump from an identified location) has been used to identify pollution sources. Sulphur isotopes have been used in soil–plant studies and used in the analysis of archaeological materials where they can provide information about ecosystems in the past, and also the diets, and movements of our ancestors. Radioactive isotopes of sulphur can also be used as tracers, for example to label DNA or RNA, and they have also been used in hydrologic studies.
This element has been known to and exploited by our forefathers for a very long time and has been mined for thousands of years. Indeed, it is likely it was made use of long before any record of its usage was ever documented.
However, some of the first records we have of it being exploited are medicinal. They come from ancient Egypt, in the form of the Ebers Papyrus (a.k.a. Papyros Ebers), a medical treatise written in hieratic Egyptian. This is dated to the reign of Amenopis I, the second Pharaoh of Egypt’s 18th dynasty, c.1,550-1,536 BC. That said, strong linguistics evidence suggests that some, if not most of the manuscript was copied from older sources. It’s thought these could date back to c.1,995-1,775 BC.

Wellcome, licensed under CC BY 4.0
This document is huge, over 68 feet in length and about a foot wide. Realistically it needed to be, since it describes nearly eight hundred and fifty herbal folk remedies, magico-religious practices, and spells. One of these explains the treatment of a condition of the eye with a paste containing powdered sulphur amongst other ingredients. This was a remedy for ‘granular eyelids’ which is probably blepharitis, although styes could be another interpretation.
Because it can be found fairly readily in its native form sulphur, as an element, is similar to copper and iron in that it wasn’t really ‘discovered’. However, the credit for its ‘discovery’ is sometimes given to an old friend of ours, Hennig Brand, the German alchemist who discovered phosphorus around 1669. Similarly, sulphur was positively identified as an element by another old friend, Antoine Lavoisier, in 1777.
But the prolific Persian alchemist Abū Mūsā Jābir ibn Ḥayyān (c. 815 AD) had actually beaten them both to it, and sulphur was one of the earliest substances to be recognised as an individual chemical element. He specifically mentioned sulphur as part of his systematic classification of chemical substances, adding both sulphur and mercury to the four classical elements of earth, air, fire, and water. In alchemical terms, sulphur provided an active principle, the male counterpart to mercury’s passive principle, and female characteristics.
Describing the nature of these elements wasn’t an entirely radical step, however. In the late 3rd-early 4th century, Greco-Egyptian alchemist, Zosimus Alchemista (a.k.a. Zosimus the Alchemist), had already professed that in effect brimstone (sulphur) was the ‘father’ and quicksilver (mercury) the ‘mother’ of alchemy.
Zosimus was the author of the oldest known books on alchemy (the ‘Cheirokmeta’), of which only fragments survive, and these mostly through translations into Syriac or Arabic. One of Zosimus’ books was actually entitled ‘The Sulphurs’. He and other early (Greco-Egyptian) alchemists believed all metals derived from common matter, indeed the concept of ‘All is One’ was common in alchemical thinking. Thus, the transformation of one metal to another was, in their view, quite possible.
Jābir ibn Ḥayyān took this a step further, and theorised (erroneously, but that’s science, never settled, ever evolving) that metals come into being by sulphur and mercury mixing in the earth. He believed that these formed, respectively, the soul and body of a metal. He further proposed that the differences we observe between metals are linked to the quality of the sulphur—for example, gold is formed from a particularly ‘subtle and well-balanced’ sulphur.
Through the history of alchemy, the characteristics that sulphur exhibits (a substance which could be ‘transmuted’ from a solid to a liquid, to a gas, and then back to a solid, whilst remaining chemically unaltered) meant it remained a fundamentally important element.
This led to it being deemed one of the ‘Tria Prima’, or Three Primes, the primary components of the universe in the 16th century, by Paracelsus, another gentleman we have met in previous articles as the ‘father of toxicology’. In this, sulphur represents the soul (of all materials and living things)—whereas mercury reflects the mind, and salt (we now know this to be a compound, but it was seen as an element by the alchemists) the body. Of our element, he wrote:
“He who does not know the sulphur knows nothing, and can accomplish nothing, neither of medicine, nor of philosophy, nor and of the secrets of nature.”
But where, you might wonder, does the name come from. Well, when it comes to the modern name, I find myself at odds with the authorities (there’s a surprise, eh?) as the International Union of Pure and Applied Chemistry (IUPAC) have decreed that the spelling for our element shall henceforth be ‘sulfur’. Unrepentant and unbowed, I make no apologies for my continued use of the name I have always regarded as correct, sulphur.
However, there are a few possible options for the origins of this name. One is said to be from the Sanskrit ‘sulvere’ or ‘shulbari’ (a.k.a. the ‘enemy of copper‘), although searching a Sanskrit dictionary I failed to locate the first of these two words. Indian alchemists were certainly aware of this element, which they called गन्धक, anglicised to ‘gandhaka’, which means the ‘smelly one’). Another possibility is that it could come from ‘sufra’ the Arabic word for yellow.
The final possibility, which I feel is the most likely root, is from southern Italy, from what is now the Campania region. Before the Romans set up camp and culturally changed the whole face of Italy, this area was home to a people known as the Osci (also called Oscans). Their homeland is part of the Campanian volcanic arc. This region is home to Solfatara, and Mount Vesuvius, and extends to include other geological hotheads, on land and undersea, on and around Sicily, like Stromboli (one of the most active volcanoes in the world), Vulcano, and Mount Etna (the largest active volcano in Europe). The word in the Oscan language for the sulphur they were all too familiar with was probably something like ‘solfa’ and this entered Latin as ‘sulpur’ or ‘sulpurium’.
This gradually became ‘sulphur’ in Latin, a word (and a spelling) which carried over into the English language, if not American English. The name is not, despite IUPAC’s diktats, universal. The Greeks called the element ‘theion‘, in Spanish it’s ‘azufre’, the Germans call it ‘schwefel’, the French say ‘soufre’, and the Italians ‘zolfo’.
But another name is commonly used for our element. This one you might be very familiar with, and it is found in the Bible. From the first instance in the Old Testament to the last in the final book of the New Testament, it occurs in some eight books and fourteen verses. The word is ‘brimstone’, meaning the ‘stone which burns’ (which it does, if ignited).
From Biblical beginnings, Genesis 19:24 speaks of brimstone to destroy the wickedness God so abhors:
“Then the Lord rained on Sodom and Gomorrah brimstone and fire from the Lord out of heaven,”
Then Revelation 20:10 speaks of judgement to come:
And the devil who deceived them was thrown into the lake of fire and brimstone, where the beast and the false prophet are also; and they will be tormented day and night forever and ever.”
Interestingly, the words ‘sulphur’ and ‘brimstone’ have such strong associations with the colour of the native element that they have entered the English language as a means to denote a yellow plant or animal. Examples include plants, such as the sulphur cosmos (Cosmos sulphureus), and the sulphur cinquefoil (Potentilla recta), and the brimstone tree (Morinda citrifolia). There are also a number of animals such as the orange sulphur, clouded sulphur, and cloudless sulphur butterflies, plus our own brimstone butterfly, the sulphur-crested cockatoo, and Herman Melville’s sulphur-bottom whale from ‘Moby Dick’ (in fact, simply a blue whale, which had picked up algal growths while passing through tropical waters).
When it comes to ancient alchemical symbols for this element, there are two commonly accepted. The one seen on the left, below, is the most commonly used symbol. It comprises an equilateral triangle on top of a Greek cross (note: a Greek cross predates Christianity and is characterised by arms of equal length).
The other symbol which is used less frequently comprises a Patriarchal cross (also called the Cross of Loraine), which has two horizontal crossbars, the upper being slightly shorter, placed on top of an ouroboros (the infinity symbol).

SharpieType301, 2023
Unfortunately, as the original meaning of so many things in our modern times has been commandeered and corrupted, this symbol for sulphur was hijacked in the 1960s. This heresy was orchestrated by a categorically creepy character, occultist Anton LaVey, who founded the Church of Satan. This led to sulphur’s second alchemical symbol, in all probability because of the link between sulphur (brimstone) and the fires of hell, becoming known as Satan’s Cross. I’d like to say that, because of this association, it has fallen out of fashion, but it seems not.
Alright, we more or less know what this element is called, but when was it first used, and for what purpose?
Sulphur and its compounds have been known since prehistoric times. Interestingly, sulphur and carbon were the only two non-metallic elements our ancestors were aware of. There has been some suggestion that elemental sulphur could have been used as a pigment for early cave paintings but, although this sounds quite feasible, I have found no concrete evidence to support this.
That said, its compounds were certainly used as pigments in ancient Egyptian wall paintings and, when mixed with a gum arabic binder, on papyri too. In particular, the rather toxic mineral ‘orpiment’ (As2S3, arsenic sulphide), was used to give a rich yellow colour, but ‘realgar’ (As4S4 or AsS, another arsenic sulphide, thus toxic) which imparts an orangey-red colour, was also used.
That the Egyptians used elemental sulphur medicinally, we have already seen from the Ebers Papyrus, but it wasn’t just a sight for sore eyes. It was also used to treat intestinal ailments (not least as a vermifuge, a de-worming agent), to relieve gout, and to lessen a fever. It is known to be helpful in treating diseases of the skin, such as eczema, and can help curb allergic skin reactions.
Back to the 17th century and right up until the mid-1900s, a medicament called ‘spring tonic’ (a mixture of blackstrap molasses and sulphur, sometimes with whiskey or herbs added) was given to children as a ‘universal remedy’. This was believed to purify the blood after the long period of winter inactivity. Realistically, it more likely acted as a laxative! However, you can still find recipes for this today, so maybe it did do a little more.
It was also known to be in use in Egypt from around 2,000 BC for fumigating buildings. Blocks of sulphur were burned, and the pungent fumes which evolved acted as a bactericide and insecticide. The Romans continued this practice, burning sulphur to fumigate the Senate House and areas which housed other public events such as amphitheatres, stadia, and circuses, to prevent the spread of disease. Since the process of burning our element produces sulphur dioxide which, in a moist environment converts to sulphuric acid, it perhaps isn’t surprising that any self-respecting bug would keel over.
The Egyptians also made use of sulphur dioxide (one of sulphur’s many compounds) to bleach cotton, a practise which seems to date to c.1,600 BC. Even to this day, sulphur dioxide is used to bleach cane sugar, to produce that white refined sugar you pop into your cuppa.
A number of ancient peoples are known to have made use of sulphur-rich hot springs for their supposed medicinal qualities. Having soaked in at least one myself, I can safely say they made me feel great. A stinky way to good health, perhaps, but why not?
Before I move on to more modern uses of our element, I must highlight a couple of ancient substances in which sulphur played a fundamental role. Both were, without doubt, historically important. Indeed, both were pretty momentous, gamechangers, in truth. Why? Well, they relate to warfare and weaponry, both of which tend to have a pretty dramatic effect on events.
The earliest of these extraordinary developments comes from Byzantium, and dates to around 673 AD. Our first sulphur-related substance was developed by a Greek-speaking Jewish (or possibly Christian—opinions differ) architect and chemist. His name was Callinicus (sometimes written as Kallinikos). This man had fled to Constantinople from Heliopolis in Syria (in modern times, this is Ba’albek, and falls within Lebanon’s borders), when his city, indeed the whole of Syria and the Levant, fell under Muslim rule following their devastating victory at the Battle of Yarmouk in 636 AD.
Having already taken flight as a refugee once, Callinicus was understandably keen for his new home not to suffer a similar fate. So, he began to experiment, combining different chemicals to design an incendiary weapon which might help keep the marauding Muslims at bay. He was unquestionably successful, and shared his secret with only one person, the Byzantine Emperor Constantine IV Pogonatus himself.
Incendiary weapons were actually nothing new in warfare. Flaming arrows had certainly been used well prior to this period as had ‘firepots’, that is ceramic vessels filled with glowing embers (or, possibly, molten pitch or tar), but Callinicus’ new weapon was something very different indeed.
What he had created was a substance that was sticky, extremely flammable, and one which, once alight, was nearly impossible to extinguish—it certainly could not be extinguished by dousing it with water. Furthermore, when it was exposed to the air (or water, according to some sources), it spontaneously burst into flames. The military advantages of this substance were clear to the emperor, and production commenced in the harbour of Galata. Its exact composition went on to be kept a closely guarded secret for centuries.
Callinicus’ substance could be used in an early form of hand grenade, by filling earthenware vessels with it, which could then be lobbed at the enemy, by hand or using a catapult. But, much more significantly, it could be used like an ancient napalm. For this, it was heated in a cauldron, stored in heated barrels under pressure, and then pumped out as a liquid through a nozzle, a ‘siphōn’ (by specially trained men called siphōnarios), to act as something like an early flame thrower. Better still, this system could be mounted on the bow of a ship!
Anna Komnene, a Byzantine princess, who wrote a history of her times, the Alexiad, a little later, described these ‘siphons’:
“Thus he [the emperor] ordered the construction on all the ships of bronze and iron heads of lions and other wild animals of all types, with open mouths and covered in gold leaf, so that their appearance alone was enough to spread fear. The liquid fire that was to attack the enemy would pass through the mouths of these heads, so that it would appear verily that they were vomiting forth flames…”
This substance became known as ‘Greek Fire’. The Byzantines themselves called it ‘liquid fire’, or ’marine fire’, and the Muslim invaders referred to it as ‘Roman fire’. But by whatever name it was called, this was a truly terrifying new weapon. Not only was the resulting inferno horrifying, but the ‘siphons’ produced a thunderous sound, like a dragon roaring, and billows of black smoke were emitted when it was employed. This made it a psychological weapon as much as a physical one.
The exact composition of Callinicus’ astounding substance remains unknown to this day, despite numerous efforts to recreate it. However, is assumed to have been a mixture of sulphur, naphtha (a mixture of flammable liquid hydrocarbons from crude oil), pitch (a viscous, dark, sticky substance from the distillation of pine resins), and possibly saltpetre (potassium nitrate) or quicklime (calcium oxide). There is every likelihood that other, as yet unidentified, components were also included.
Anna Komnene once again comments on the composition of this substance but, since she was a Byzantine emperor’s daughter, it is very likely she shaded the truth somewhat to conceal the secret of this precious superweapon. Nevertheless, she explains:
“This fire is made by the following arts. From the pines and the certain such evergreen trees inflammable resin is collected. This is rubbed with sulphur and put into tubes of reed and is blowing by men using it with violent and continuous breath. Then in this manner it meets the fire on the tip and catches light and falls like a fiery whirlwind on the faces of the enemies.”
It was first used militarily, and to most shocking effect, against the Saracen armada who’d laid siege to the city of Constantinople in 677 AD. To put it mildly, the Muslim forces, under the command of Muʿāwiya ibn Abī Sufyān, founder and first caliph of the Umayyad Caliphate, received a rather unpleasant surprise.

Codex Skylitzes Matritensis, Public Domain
Muʿāwiya’s flotilla was facing off against a much smaller Byzantine fleet, and they must have been confident that victory was surely theirs. But then, from the bows of the Byzantine ships, they’ll have seen small bronze tubes projecting. To their astonishment and horror, from these, jets of liquid fire were turned onto the unfortunate enemy. Everything the fire touched, it stuck to, and all was engulfed in flames. Even those poor souls who jumped or fell overboard to escape the inferno were not safe. The fire spread across the surface of the water like a blazing raft and continued to burn (apparently even under the surface), annihilating men and vessels alike.
Why, one might wonder, did the Byzantine vessels not suffer a similar fate? Well sometimes they did, but in an effort to protect them, the Byzantine ships had been coated in a special mixture to render them resistant to fire. This was said to include vinegar, alum (hydrated sulphate salts of aluminium), and talc (hydrated magnesium silicate).
Time and again the Byzantines went on to use their ‘liquid fire’ with great success in naval battles, and they adapted their methods so it could also be used on land. This new weapon really was a gamechanger—indeed, just the sight of a ‘siphōn’ stuck terror into the enemy. A formidable deterrent indeed. The secret formula was preserved by each of the Byzantine emperors in turn, being handed down only to their successors for over seven centuries. This extraordinary military advantage was probably one reason the Byzantine empire lasted as long as it did.
But sulphur plays a most important part in another substance which, whilst it is a slightly newer innovation than Greek Fire, has had a much longer lasting and far-reaching impact, even today. This substance is gunpowder.
Whilst its precise origins are lost to the mists of time, it is certain that gunpowder had been developed in China by the late Tang dynasty (7th to 10th century AD). It’s thought that it was probably discovered by accident, by a Chinese alchemist searching for the secrets of eternal life. It doubtless came to his attention (in a very noticeable fashion) when his mixture was exposed to a flame, and didn’t just ignite, but went boom! I would imagine that would have been a moment he never forgot.
It has been proposed that the origins could, in fact, be a great deal earlier than the Tang dynasty, since a manuscript written by the alchemist Wei Boyang in 142 AD (the Cantong qi, 參同契, or the Book of the Kinship of Three) refers to a mixture of three powders which “fly and dance” violently.
In addition, in 318 AD, the ingredients of what we now know as gunpowder are recorded in the Baopuzi (抱樸子, a.k.a. The Master Who Embraces Simplicity), written by Taoist philosopher Ge Hong. He listed saltpetre (potassium nitrate), pine resin, and charcoal as components of the mixture.
Although sulphur was not specifically mentioned in the documents above, it most certainly was by 808 AD. Then, Taoist priest Qing Xuzi, listed it in the Taishang Shengzu Jindan Mijue (太上聖祖金丹秘訣), as part of huo yao (so called ‘fire medicine’).
Furthermore, in 858 AD, the text Zhenyuan miaodao yaolüe (真元妙道要略, a.k.a. Classified Essentials of the Mysterious Way of the True Origin of Things) carries a caution about the dangers of this alarming substance, stating that:
“Some have heated together sulphur, realgar (arsenic disulphide), and saltpetre with honey; smoke [and flames] result, so that their hands and faces have been burnt, and even the whole house burned down.”
This explosive substance seems, initially, have been regarded as a bit of a novelty, possibly being used in religious ceremonies as the popping and crackling noise, made when it was sprinkled onto a fire, was thought to drive away demons. It may also have found an early use in fireworks, for which the Chinese are rightly famous.
However, its military significance soon became apparent. Over the next century, there were numerous developments in Chinese military uses for gunpowder. These ranged from ‘fire arrows’, projectiles comprising of a pouch of gunpowder attached to an arrow’s shaft, to ‘slow match’ detonation cords, to ‘fire lances’ a bamboo tube filled with gunpowder with a slow match, which was bound to a spear. These could be used on their own, to blast a stream of flame towards the holder’s opponent or could be adapted to fire broken pot shards or iron pellets.
Gunpowder not only found applications in these proto-firearms, but in other weaponry too. It could be, and was, used as a propellant in rockets and an explosive in early grenades and bombs.
By 1298 AD, gun manufacture in China appears to have been a very well-established process. Evidence for this comes from the Shangdu huochong (a.k.a. ‘Xanadu Gun’), a small bronze hand cannon, the oldest surviving firearm to bear a verifiable production date and serial number.

Aldermanseven, licensed under CC BY-SA 4.0
In the centuries to follow, weapons based on gunpowder began to appear in other parts of the world, from Arabia, through Europe, and in India, its secret having escaped from China.
Documented formulae for gunpowder (also called ‘black powder’) are known from the 13th to 16th century Mamluk Sultanate (the state which ruled Egypt, the Levant and western Arabia). Syrian chemist and engineer Hasan al-Rammah was known to have experimented with explosive substances and designed weapons to use ‘black powder’, including the first torpedo.
The Mongol Empire, established by Temüjin (Genghis Khan) and expanded by his successors, is considered by some to be the first ‘gunpowder empire’, and they were likely responsible for the spread of the secrets of this fearsome substance across the various countries they either conquered or invaded. Indeed, in India, the first documented use of this substance comes from the Mongols who, under the command of Ögedei Khagan (Temüjin’s son), invaded the subcontinent in 1221 to 1327 AD.
Meanwhile, in England, the English philosopher Roger Bacon was certainly aware of this substance, if not its potential applications. He recorded an encrypted formula for gunpowder (used in firecrackers) in 1249, in his ‘Epistola de Secretis Operibus Artis et Naturae, et de Nullitate Magiae’, whilst his ‘Opus Majus’ stated:
“We have an example of these things (that act on the senses) in [the sound and fire of] that children’s toy which is made in many [diverse] parts of the world; i.e., a device no bigger than one’s thumb. From the violence of that salt called saltpetre [together with sulphur and willow charcoal, combined into a powder] so horrible a sound is made by the bursting of a thing so small, no more than a bit of parchment [containing it], that we find [the ear assaulted by a noise] exceeding the roar of strong thunder, and a flash brighter than the most brilliant lightning.”

Ashley Van Haeften, Public Domain
But military minds soon put two and two together. By the early part of the 1300s, gunpowder was being produced by hand (using imported materials) in the Tower of London. By the 1320s, guns in Europe began to spread like wildfire, with new developments at every turn. At this stage, I will pass the baton to our good friend Tom Pudding, whose knowledge of early firearms and their development will far outstrip mine.
It wasn’t only used in weaponry though. Gunpowder also found other applications and was used to blast through rock in mining and civil engineering projects around the world until somewhat safer alternatives were developed.
Aside from its inclusion in gunpowder, sulphur has found a role in warfare in other ways, even as recently as the Napoleonic Wars. In 1812, a cunning plan was conceived by Captain Thomas Cochrane, Earl of Dundonald, to employ ’sulphur ships’ or ’stink vessels’ against the French. These ships were to be strengthened old hulks, the upper deck layered with charcoal and sulphur. When the charcoal was ignited, clouds of what Cochrane called “noxious effluvia” would be given off. The choking sulphur fumes would overwhelm the enemy’s ships and forts, giving the British the advantage.
He shared this plan with the Prince Regent (later King George IV) who sent it to a panel of ‘experts’, but it was put aside as they felt it ‘too inhumane’ to be put into use. Ironically, this demonstration of British reserve and ethical standing (remember, we used to have that) came months before the first use of non-sulphur-related poison gas in warfare during the Battle of Humin-Bolimów when German forces fired some 18,000 so-called ‘T-shells’ (T-Granate, tear gas shells) loaded with xylyl bromide on Russian positions west of Warsaw.
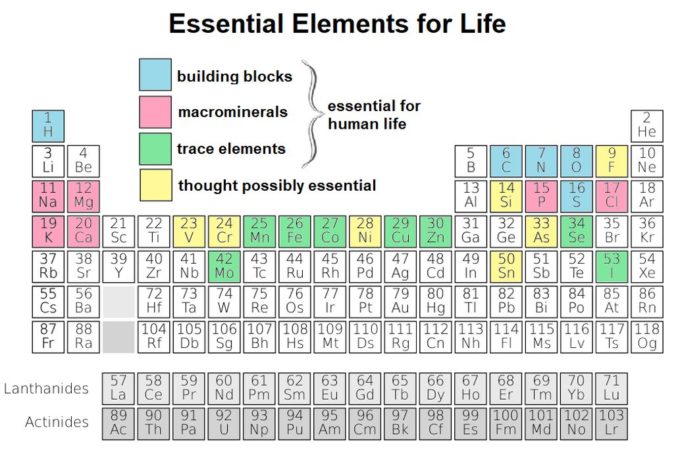
But, enough of death and destruction. That’s not all sulphur provides. It is also present in all living plant and animal tissues. Indeed, it is classed as one of the main building blocks of life (in blue below) and makes up about 0.3% of the human body.
SharpieType301, 2023, adapted from Cepheus, Public domain
It is a component of some proteins, but we cannot produce it within our bodies. As such, it is an essential part of the human diet. It’s found in protein-rich foods: eggs, meats, especially organ meats, seafood, and dairy products. It is also found in many plants, for example, broccoli and other brassicas, onions, garlic, nuts, legumes, and (whispers this bit) soy products.
It protects against cellular damage and oxidative stress, and helps cells utilise oxygen. It has anti-inflammatory, and antimicrobial as well as antioxidant effects. Thus, it helps keep our bodies healthy, resistant to infection. It plays an important role in insulin production, supports cardiovascular function, and may help protect against cancer.
Since it is used in the production of some amino acids and proteins (and the DNA that contains the instructions to make proteins), it helps ensure the health of our muscles, joints, tendons and ligaments, bone, skin, hair, and nails. Because of this, it’s sometimes called the ‘beauty mineral’ but, interestingly, a number of sulphur compounds (notably sodium sulphide, sulphuric acid, and chromium sulphate) was also used in processes to tan leather in the past. Make of that what you will.
Sulphur compounds are commonly used in winemaking. Firstly, powdered sulphur is dusted onto the growing vines to discourage the dreaded fungal disease ‘powdery mildew’ (Erysiphe necator). Later, once the grapes have been harvested, sulphur dioxide (SO2) is used at a number of stages through the process to prevent the wine oxidising and to inhibit the growth of undesirable yeasts and bacteria. Some wineries even burn sulphur candles to maintain the purify of their cellars.
Sulphur compounds are responsible for making you tear up when you cut onions, and for making your wee smell odd after you’ve eaten asparagus. Oh yes, and you know that delightful aroma the world experiences after you’d overdone the jungle juice and curry? That foul flatulent fragrance will be from the sulphurous compounds (mostly hydrogen sulphide, H2S), your digestive system is trying to dispose of. Don’t worry though, even at your worst you won’t out-stink a skunk. They use sulphur to protect themselves from attack. The sulphur-based spray they employ to such memorable effect contains two smelly sulphur compounds (tert-butyl mercaptan, (CH3)3C-SH, and thiocresol, CH3-C6H4SH) and is powerful enough to repel bears!
Industrially, sulphur’s major use is to produce sulphuric acid (H2SO4), as a reagent in its own right, but more usually used as a precursor or intermediate in the production of many other compounds for the chemical and pharmaceutical industries. Another major use for sulphuric acid is to produce phosphate, nitrogen, potassium, and sulphate fertilisers.
Sulphur and sulphuric acid also play a part in the manufacture of non-ferrous metals, fibres, pigments, detergents, and, perhaps surprisingly, in cosmetics, and personal care products. In addition, they are used in lead-acid car batteries, as part of the oil refinery process, to extract some minerals, in the vulcanisation of rubber and synthetic alternatives, and in the making of matches. Sulphur compounds are added to natural gas, which is naturally odourless, so that leaks can be detected.
We’re coming near to the end of sulphur’s tale, but I just want to talk about a couple more things. One of these is to take a look at the processes by which sulphur is procured.
These days, there are a few methods we can use to obtain sulphur. One of these, which provides about half of the annual amount of sulphur required, is as a by-product of the various processes used by oil and gas refineries. These ensure crude oil and natural gas is ‘desulphurised’ to improve its quality.
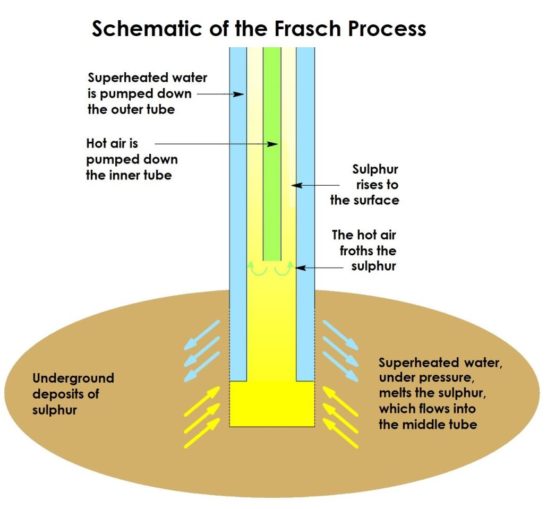
Another method is to extract the sulphur from underground deposits (there are huge deposits of near-pure sulphur, along the US Gulf coast, in Poland, and in Sicily) using the Frasch Process. This dates to 1894, when it was developed by a German-born American chemist, Herman Frasch. He first used it successfully in Louisiana, in an area which, because of its geology, had been loath to permit conventional mining practices.
Adapted from Rifleman 82, Public Domain
The Frasch Process takes advantage of the fact that sulphur melts at a relatively low temperature (115.21°C). So, superheated water, at 165 °C, can be used to melt the element deep in the earth below, and a combination of this and hot air pumped in to help it move freely, drives it to the surface where it can be collected. This process provides a very pure form of the element (c. 99.7 – 99.8% purity), with little contamination. However, this technique is limited to deposits which occur in permeable layers of rock trapped between layers which are impermeable.
There are other ways to obtain sulphur. Historically, a variety of iterations of a technique called the ‘Sicilian method’ were used prior to Frasch’s innovation, and all relied on melting the sulphur. These weren’t particularly efficient, and the pollution associated with them (in the form of sulphur dioxide, SO2, a precursor to acid rain) were problematic. Furthermore, the sulphur produced wasn’t particularly pure.
This was because the sulphur was not easily separated from the rocks in which it was present. These needed to be ‘roasted’, heated to extract the sulphur from a variety of sulphur ‘ores, including limestone calcite (> 90% of the ores used), calcitic dolomite, clay, gypsum, opalite, and quartzite. These ores were mined, across the world, typically from quarries rather than underground seams, although so-called ‘borehole mines’ were also used.
This was (and actually still is) a truly horrendous job. The sulphides exposed as the ores are mined react with the air, and moisture, to produce sulphuric acid which can, if it becomes concentrated, cause severe burns and terrible injuries. Even moderately concentrated, the fumes from this will immediately affect the miners. Any standing water becomes acidic, causing more problems since, if a miner is burned, the water they’d usually be able to use to relieve their pain will be acidified. This corrosive environment is just one of the hazards. The usual hazards associated with any type of mining are still there, including that ‘stinkdamp’ (hydrogen sulphide, H2S), which is highly poisonous, and also flammable. Could this be the most dangerous job a man could do?
No, actually. It gets worse because sulphur can also be ‘mined’ in its elemental form, and has been, as far back as prehistoric times. This was the most commonplace method in the past, in areas where the source is within the craters of active volcanoes.
Indeed, until relatively recently (late 19th to early 20th century), sulphur was mined in volcanic regions across Italy, New Zealand, and Chile, amongst other areas. In fact, New Zealand’s White Island (a.k.a. Te Puia Whakaari, which means ‘the dramatic volcano’) mine only ceased production in the 1930s. This is the same volcanic island, you might recall, which erupted unexpectedly in 2019, killing twenty-two tourists, and injuring another twenty-five more, some critically. I think this gives you an idea of some of the additional hazards.
The immensely dangerous practice of mining sulphur from active fumaroles still goes on (albeit at a much-reduced scale) today in Indonesia. Several volcanos, including the Kawah Ijen Volcano, are mined, with workers as young as twelve being exposed to the deadly conditions. Even to begin their working day, the miners have to clamber up the flanks of the volcano, then down the precipitously steep walls of the caldera to pry away chunks of solidified sulphur.
They carry what they collect, often weighing more than they do, back out of the volcano up the sheer, unstable pathways to the caldera’s rim. Losing one’s footing here is not to be recommended as the volcano is also home to the world’s largest highly acidic lake, which lies at the base of the caldera. The pH of these ‘waters’ have been measured as low as pH 0.5. That’s the equivalent of a lake of battery acid!

Okkisafire, licensed under CC BY-SA 3.0, and Candra Firmansyah, licensed under CC BY-SA 4.0
As the photographer of the second of the images above reports:
“This image shows the dangerous and rugged conditions the miners face, including toxic smoke and high drops, as well as their lack of protective equipment. The pipes over which they are standing serve to guide sulphur vapours and condense them, thereby easing (well, relatively at least) production. What looks like clouds or steam is actually highly concentrated hydrogen sulphide and sulphur dioxide gases.”
There is a 2008 video of sulphur mining being carried out at the Gunung Welirang Volcano, East Java, Indonesia, which gives some idea of the conditions faced by the miners.
Sulphur’s story, as one might expect for an element with cultural connotations linking brimstone with Satan and the underworld has been a depressingly damaging one. After considering its uses in warfare and the perils of obtaining this substance, I would rather close this piece on a more pleasant note, something sulphur-related, but beautiful.
When we explored copper, I mentioned a couple of gloriously coloured minerals which have long been used as semi-precious gemstones and pigments. In a comment which, of course, no-one pays any attention to, our friend Chrissie mentioned that she loved these semi-precious stones, and I hinted that there may be another one to discuss.
There is indeed, and it I think is one of the most beautiful. It has been highly prized since the ancient civilisations of Mesopotamia, Egypt, China, Greece, and Rome, and is to this day for its gloriously rich blue colour. The mineral (actually a stone) is lapis lazuli, which is, in fact, a mixture of three minerals: lazurite, calcite, and pyrite.

Grendelkhan, licensed under CC BY-SA 3.0
The lazurite (with a chemical formula of (Na,Ca)8(AlSiO4)6(S,Cl,SO4,OH)2) is responsible for the gorgeous bright royal blue colour. It is actually the trisulphur radical ion S3•−, which give it the intense blue. The calcite gives the stone attractive streaks or bands of a milky white, but the inclusion of gold-like flecks of pyrites gives the stone its sparkle. This has been compared, even by Pliny, to stars shining in a midnight sky.
One of the primary sources for this stunning stone (which, when ground to powder, is also the source of the pigment ultramarine) is the Kokcha River valley of Badakshan, a province in north-eastern Afghanistan. This source has been exploited since the Neolithic, c. 3,500 BC, even before it became known as Bactria (yep, the place after which those splendidly shaggy two-humped camels are named). There are lesser quality deposits in other parts of the world, but Afghan lapis is still the most highly sought.
I shall leave the very last word to a familiar and much-loved author, Rudyard Kipling, who knew that country well, and who commented:

Adapted from Leslie Ward – Vanity Fair, Public Domain
© SharpieType301 2023



