Towards the end of the 19th century, scientists were considering the structure of the atom. The atom was deemed to be a tiny piece of matter which helped answer questions regarding spectroscopy. Spectroscopy being the interaction between matter and electromagnetic radiation which includes light. Atoms of different material absorb and emit different wavelengths of electromagnetic radiation – this was how helium was detected in the Sun before it was detected on Earth hence its name originating from Helos, the Sun.
J. J. Thomson believed the atom to be an indivisible piece of matter which, owing to the earlier discovery that electrons have a negative charge, would be made up of a physically larger substrate that was positively charged – it had been suggested that atoms were electrically neutral and the electrons would cancel out the charge of the positive substrate. The electrons in Thomson’s model were thought to orbit within the substrate and so the model became known as the ‘plum pudding’ model. This model had a couple of problems and the arguments against it would not go away. To begin with, anything in circular motion is in a state of constant acceleration. To clear up any confusion, acceleration is defined as a change in velocity (over time) where velocity is defined as a speed with a direction. This means that a change in direction without a change in speed can be called acceleration. So for electrons to orbit within a sphere, they would need to constantly change direction so as not to fly out of the sphere and are therefore in a state of constant acceleration. All of this requires energy.
Secondly, any acceleration of a charged particle like an electron will emit radiation of some type but, with the exception of some, atoms do not emit radiation.
These problems did not stop the theory becoming accepted in many quarters as it mostly fitted the bill and worked with the Periodic Table nicely however not everybody was a believer.
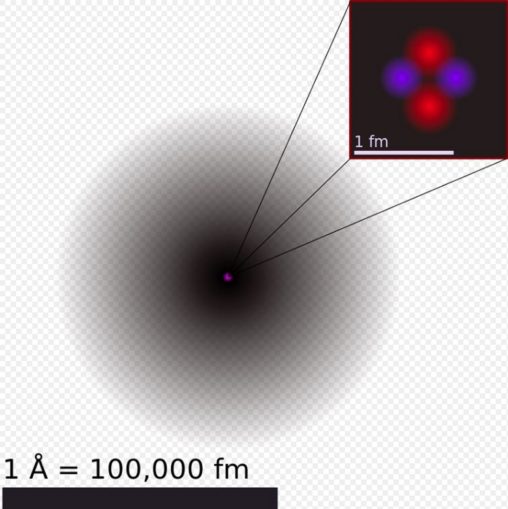
User:Yzmo [CC BY-SA]
If the plum pudding model is correct then with the alpha particle being 7000 times the mass of an electron, a collision with a stray electron would have no effect even considering the electrostatic repulsion of a negatively charged electron and a positively charged alpha particle.
If the alpha particle collided with the positively charged substrate then at worst and given the high velocity of the alpha particle, it would do no more than deflect it whilst it continues its journey through the gold foil – ricocheting between the plums until it exits.
Sure enough, this is what was found, alpha particles weadling their way through the foil but then Rutherford asked Marsden (who by then asked a colleague called Hans Geiger to assist) to stop looking at alpha particles going through the foil but instead look for alpha particles that would be deflected back from the foil towards the point of origin. When this was done, it was noticed that a number were indeed reflected back. Granted, not a large number but nevertheless, a number that could not be ignored so what was going on?
This result helped Rutherford complete his theory. What was happening was that most alpha particles would happily skim past the atoms in the gold foil because, as had been suggested, atoms were largely made up of empty space. Should an alpha particle hit an electron belonging to a gold atom then, like a bowling ball hitting a table-tennis ball, it would plow on through. If an alpha particle was seen to be reflected back then this was the alpha particle colliding head on with the nucleus of a gold atom. Yes, there were obviously some variation in the angle of deflection but it depends where it ‘hit’ the gold nucleus so it was to be expected. It should be noted that the alpha particles and gold nucleus do not physically collide, both are positively charged and so electrostatic repulsion replaces an actual collision.
This single experiment should have put paid to the plum pudding model but, like the facts not fitting the theory of man made climate change today, many scientists decided that the evidence before them was wrong and for good reason….except that they couldn’t think of one.
1 year later in 1911, Rutherford put together his model of the atom which had a central nucleus that was entirely positive in charge and had electrons orbiting outside this nucleus. Rutherford made a number of predictions based on his atomic model which were all confirmed in an experiment by Geiger and Marsden in 1913.
3D animation of an atom incorporating the Rutherford model
This is when scientists began to take more notice, especially a Danish physicist called Nils Bohr. Bohr combined Rutherford’s atomic model with ideas on quantisation from Max Plank whilst studying the most basic atom of all, the hydrogen atom. These ideas were the basis of quantum physics and were finally fed back in to the study of spectroscopy (this is where we came in) which allowed predictions to be made and eventually found to exist.
Bohr went on to refine Rutherford’s model of the atom and just as importantly, face head on one of the problems associated with acceleration of the orbiting electrons which had dogged previous models.
Yes, the electrons should spiral in to the nucleus of an atom if the electron receives no more energy to maintain its orbit however, based on Plack’s quantum hypothesis, the electron can only move in orbits where its angular momentum is an integer multiple of ħ (where ħ = h/(2π) and h is Planck’s constant). In such an orbit, the electron can orbit indefinitely and will emit no radiation.
Advances in the quantum model mean that we have now also moved on from the Bohr model of the atom though this is still the model taught in schools. The orbits of electrons are not so much orbits as spheres of probability. This means that, owing to Heisenberg’s uncertainty principle, we can’t know the orbit precisely however, we can create a cloud of probability in which, the electron will definitely be located. This means that atoms look a little different to the way that we have come to know them but that is the idea of a model – to simplify a concept to enable it to be worked on without losing important detail.
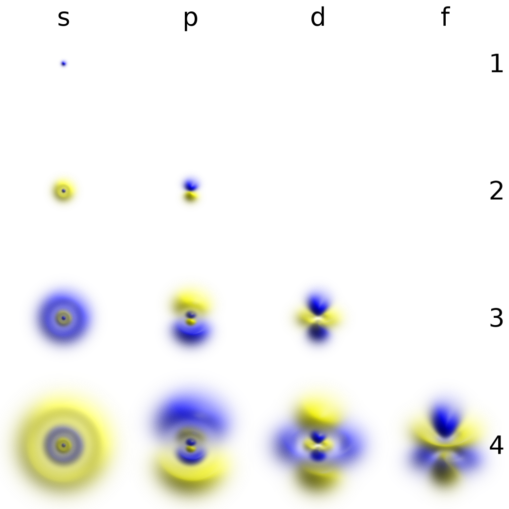
Geek3 [CC BY-SA]
The above are the clouds of probability for each electron shell. These shapes are combined for larger atoms and can give some very impressive representations of atoms – atoms are not 2 dimensional!
© Rat Catcher 2017



