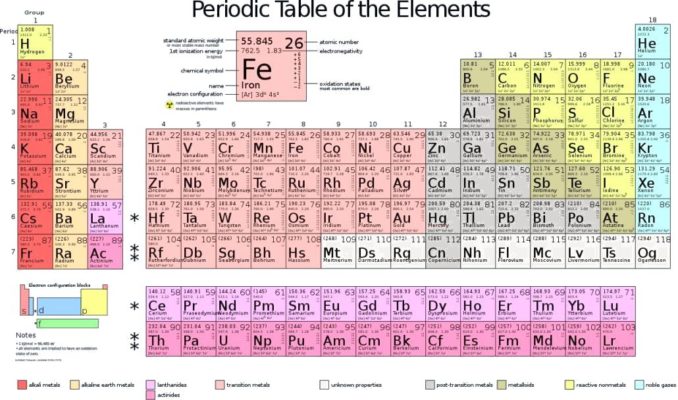
Behold the Periodic Table (a.k.a. the Periodic Table of the Elements). I’m pretty sure that we are all pretty familiar with this, from happy days in the school classroom if not more recently.
2012rc, licensed under CC BY 3.0
It’s an interesting structure in its own right, so I’ll start by having a look at the table, how it came to be and why it looks as it does. However, in subsequent pieces, I thought it might be fun to have a more in-depth look at the story behind some of the stars of the coloured squares, the elements themselves.
This series certainly won’t cover each and every one of the elements included in the Table to date—there are currently 118 of these, of which only 94 occur naturally (the remainder being synthesised). It’ll be somewhat ad hoc, so nor will it necessarily be written in any discernible order. I’ll simply pick up the elements, or groups of them, I feel inclined to write about at the time. Feel free to request any favourites. I might include ‘em but then again, I might suggest that you write an article of your own.
I may include one of the new kids on the block if any new-fangled ones pop up and are given names. That said, they are relatively boring, so for the most part I’ll cover elements most of us will be familiar with. American mathematician, satirist, and musician, Tom Lehrer gives us a nice recognisable list in his song, ‘The Elements’, this recording filmed live in Copenhagen in 1967.
Before I kick off to look at why they were lined up into a table in the first place, let’s have a little bit of revision to remind ourselves what an element actually is.
The Britannica Dictionary defines an element as:
“one of the basic substances that are made of atoms of only one kind and that cannot be separated by ordinary chemical means into simpler substances.”
Ah, OK, thanks. That’s clear as mud then. What the hell is an atom?
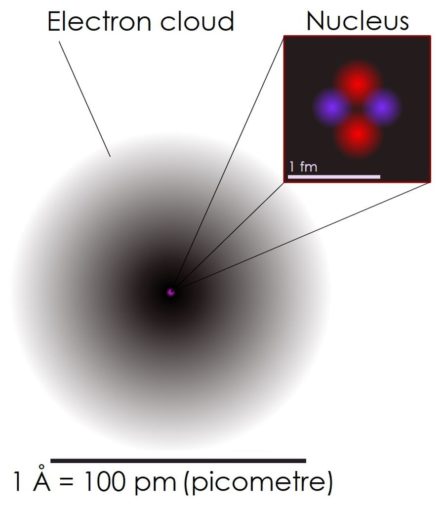
Well, I like to think of an atom a bit like a Lego piece. They are the little building blocks which make up all ‘matter’, or as I prefer to think of it, ‘stuff’, whether that be a solid, liquid or gas. Oh, and plasma, sometimes described as the fourth state in which matter can be seen.
Atoms are extremely small, but as little as they are, atoms aren’t the tiniest thing known to man. Atoms are made up of three principal subatomic particles. These are protons (which carry a positive charge) and neutrons (that have no charge), both of which sit at the core of the atom called the nucleus, and electrons (with a negative charge) which hang around in a loosely arranged ‘cloud’ around the nucleus, attracted by the positive charge of the proton, not too dissimilar to Puffins when the bar is open.
Yzmo, licensed under CC BY-SA 3.0
In reality, the make-up of atoms gets a whole lot more complex than this. Although the electrons have no known subcomponents, both protons and neutrons are awkward little beggars, being composite particles made up of miniscule subatomic particles called quarks. Those who are keen to descend further into this particular rabbit hole may like to know that six different types of quarks exist, these being identified as ‘flavours’ called: up (u quark), down (d quark), charm (c quark), strange (s quark), top (or truth quark), and bottom (b quark).
These subatomic particles pre-date the emergence of atoms in the history of Life, the Universe and Everything but I’ll just leave this here; this really is an article for someone else to write!
As a slight aside, you’ll also hear people bandying about terms such as molecules and compounds as well as elements and atoms. What’s the difference?
Well, we now know what an atom is, and an element too, but a what is a molecule?
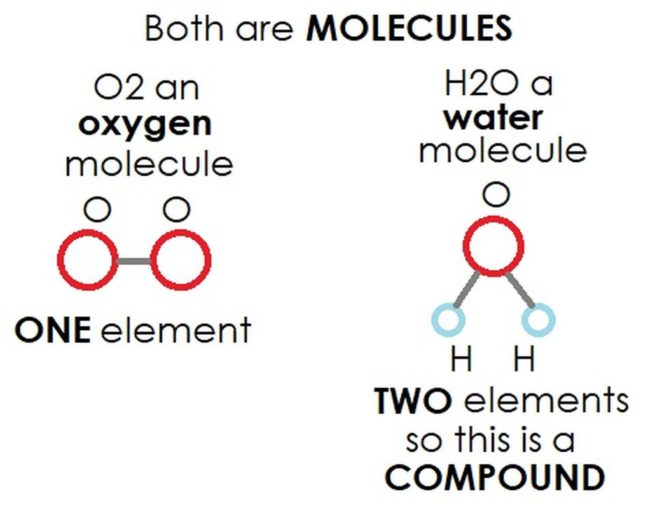
Molecules take shape when two or more atoms form chemical bonds, which bind them together. The atoms which are bonded together can all be the same element.
O2 is an oxygen molecule made up of two atoms of the element oxygen, O, and an ozone molecule, O3, is made up of three atoms of the element oxygen.
Alternatively, a molecule can be made up of more than one element.
H2O, water, a molecule of which has a single atom of the element oxygen, O, bonded to two atoms of the element hydrogen, H.
SharpieType301 2022
If more than one element is bonded in this way, it is still a molecule, but is also referred to as a compound (i.e., a combination). Compounds can be quite simple, like H2O (water), or can be much more complex, like C8H10N4O2 (caffeine). Hmmm, before I go much further, this might be a good time to pour a cup of Joe.
In summary, all compounds are molecules, but not all molecules are compounds.
OK, so an element is a bit of matter comprising one or more atoms of the same ‘stuff’. Great, got it.
But how do we identify which element we’re interested in? Well, each element will have:
- its own name (e.g., carbon)
- a unique chemical symbol which will comprise one or two letters, the first of which is capitalised (in carbon’s case, this is C)
- an atomic number indicating the number of protons in its nucleus (in the case of carbon, 6).
A Periodic Table will always show these three things, though it may also provide further information about each element, such as its atomic mass (a.k.a. atomic weight), which group it falls into (e.g., non-metals, alkali metals, transition metals, noble gases, etc.), the mass number (how many protons and neutrons it has), and other bits and bobs of interest to a relative minority of science geeks.
Now let’s have a look at the table itself.
As we see it today, the Periodic Table was last revised in 2016. As things change in science (it is without a doubt not settled), new elements are occasionally synthesised and given their place of honour in this fascinating graphical representation. The latest of these was synthesised in 2010 and was assigned the name Tennessine. A real heavyweight, atomic number 117 (with those 117 protons in its nucleus), this has been allotted the symbol Ts.
The Periodic Table we saw right at the start of this piece is arranged in rows called periods (hence the ‘periodic’ table), read from left to right in order as the atomic number of each element increases. The vertical columns are called groups. These correspond to ‘groups’ or families of elements which display similar properties. All elements have specific physical and chemical properties, but many will display similar properties. Since these tend to occur at regular intervals, this gives us the grid structure of the Periodic Table we are most familiar with.
The history of this table of the elements is deserving of an article in its own right, not least because what we see today was a decidedly long time in the making. It reflects the combined efforts of many scientists, although one could say that it actually began with the Ancient Greeks.
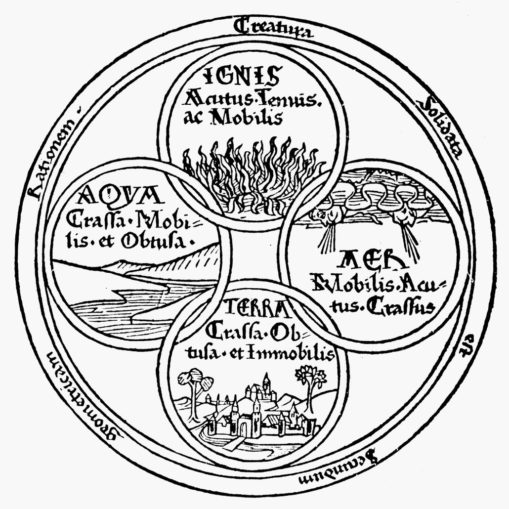
Prior to the philosopher Empedocles’ hypothesis about what an ‘element’ actually was there were nine materials that we’d now call chemical elements known to our ancestors. These were substances found in their native form, mostly metals: iron, copper, silver, tin, gold, lead, and mercury, although there were two additional non-metallic elements known: carbon, and sulphur. Around 450 BC, Empedocles proposed that ‘elements’ were simple, everlasting, and unchangeable, and that any variation was merely the result of their combination and division.
A bit of an oddball, our Empedocles, who really did hold himself in high esteem. There’s a legend that he died by throwing himself into mouth of the volcano on Mount Etna in Sicily, so that people would think he’d simply vanished to become an immortal god. However, the Greeks gave credence to Empedocles’ notion that all matter (including the native form elements above) were formed or could be formed by combining one or more of his four main ‘roots’ or classical elements: air, earth, fire, and water, in different proportions.
Unknown author, public domain
Yet rather than Empedocles, it was philosopher Plato who coined the term ‘element’ (στοιχεῖον), and it was his pupil Aristotle who firmly established this concept to describe the physical world, one which remained doctrine for centuries.
Born in 384 BC, in what is now Central Macedonia in Northern Greece, Aristotle was appointed to tutor the young Alexander (later to become Alexander the Great) by his father, Philip II of Macedon. Aristotle had developed a process of logical deduction (syllogism) and applied this logical approach to the many subjects he both studied and taught. Aristotle’s view of the four elements (though he believed that there was also a fifth element, the quintessence, a divine substance known as aether) persisted through the history of alchemy (a protoscience which sowed the seeds for modern chemistry), right up to its Golden Age in the 1600-1700s.
Be that as it may, other elements were known to the ancients, but were not necessarily recognised as such. The metal antimony (symbol Sb), sometimes found in native form, was powdered for use in cosmetics (the black eye makeup used by Queen Jezebel in the Old Testament was antimony based), and in medicines (it was used as a laxative) as far back as 800 BC, perhaps longer. With regards to the latter, please don’t try this at home! The metalloid arsenic (symbol As), though it rarely occurs as a pure element, was used by the Mesopotamians in metallurgy around 4000 BC and was known as a drug and as a poison by around 2000 BC. In fact, its name derives from ἀρσενῐκόν (arsenikon), meaning ‘potent’ or possibly ‘male, virile’.
in 1669 the German alchemist Hennig Brand accidentally discovered a new chemical element while pursuing the legendary ‘philosophers’ stone’. By now at a time when written records were kept, we know a little more about this.
The ‘philosophers’ stone’ was of huge importance to the alchemists. A mythical substance, it was believed to be able to ‘transmute’ base metals into gold. It was also considered the ‘elixir of life’: thought to cure all ills, promote rejuvenation, and give the user eternal youth or immortality (hokum, eh, though I do believe that the quest for such an ‘elixir’ may yet be ongoing in our supposedly enlightened age).
Brand was experimenting with a charming substance, concentrated urine (boy I’m glad I never worked in his lab!). He combined this with other materials, heating it until he produced fumes which glowed as if with an inner light and droplets of a liquid which he caught in a jar. When covered this lustrous liquid solidified and, for a time, the pale-green glow persisted.

Derby Museum and Art Gallery, public domain
Hey, he was surely onto something great here. Well, yes, but not quite in the way he thought. Brand named this substance ‘light-bearer’ (phosphorus, or in Greek, Φωσφόρος) because of this extraordinary property. From this, comes ‘phosphorescence’. What he’d discovered was an extremely reactive element, never found as a native or free element because of its reactivity, but always as part of a compound. This has atomic number 15, and was later given the symbol P.
But by the 18th century, what we would consider to be the beginnings of modern chemistry took shape, and such ‘ancient wisdoms’ were gradually being edged out. One of the first steps in this process, and in the journey towards the Periodic Table, came as the result of work by the French nobleman and chemist Antoine-Laurent de Lavoisier.
A prolific scientist, he developed a rigorous system of chemical nomenclature and helped turn what was largely a qualitative practice into a quantitative one. In 1789, in his Traité Élémentaire de Chimie (Elementary Treatise of Chemistry), the first modern chemical textbook, he listed substances that could not be broken down further (i.e., elements), classified the known elements by their properties and grouped these substances as gases, earths, metals, and non-metals. We will see more of this extraordinarily man in future episodes.
Lavoisier co-operated with a number of other early chemists, but perhaps the greatest influence on his work came from the young noblewoman who was to become his wife, Marie-Anne Paulze.

Jorge Elías, licensed under CC BY 2.0
She was a well-educated young woman, and a skilled artist. Quite a force in her own right, she was a mere thirteen years of age to his twenty-eight when they married. She translated scientific papers from English to French and critiqued these for him, illustrated his work with her sketches and engravings, hosted gatherings at which eminent scientists could debate their theories, and acted as both his lab assistant and the editor of his reports and publications.
Regrettably, this was not a time to be of noble origins in France, however philanthropic he had been in opening up access to others in their scientific endeavours. The Terrors of the French Revolution put an end to the work the Lavoisiers were engaged in, and Antoine was sent to an appointment with Madame la Guillotine in Paris in May 1794. Just a short while later, towards the end of 1795, he was found innocent of the trumped-up charges which had been levelled against him, and completely exonerated. Joseph-Louis Lagrange, Italian mathematician, physicist, and astronomer, mourned this huge loss to science, saying of Lavoisier that “It took them only an instant to cut off that head, and a hundred years may not produce another like it.”
As new elements were discovered over the years, a number of theories emerged in an attempt to classify them. Arguably the next significant move was made by German chemist, Johann Wolfgang Döbereiner. You might want to remember this man’s name the next time you reach for a cigarette. As part of his work identifying the catalytic properties of platinum, he is also credited with the design of the first portable gas lighter. This became known as a Feuerzeug or Döbereiner’s lamp, a remarkable invention which pre-dates the development of friction matches. OK, it was designed to light candles, not your Benson & Hedges, but you get the drift.
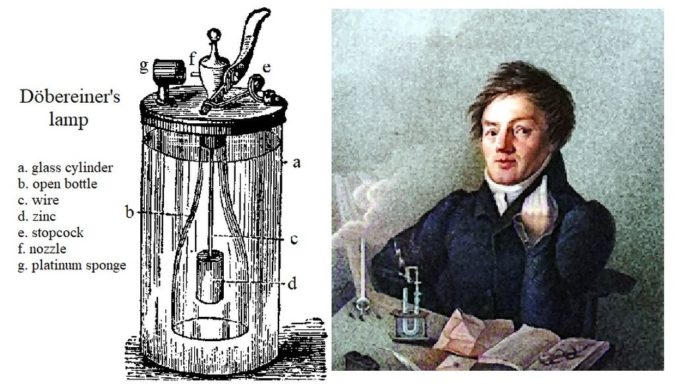
Pixel 17, licensed under CC BY-SA 2.0
With none of the societal privileges that the Lavoisiers were born to, in 1794 at fourteen years old Johann’s mother apprenticed him to the local apothecary (an early pharmacist). A bright lad, this experience, along with his independent studies, proved invaluable and the knowledge he accumulated allowed him to attend the University of Jena where, very much a self-made man, he later became a professor of chemistry and pharmacy.
Döbereiner realised that, when the fifty-three elements which had been identified at this time were arranged in increasing order according to their atomic mass, the chemical and physical properties of certain elements seem to lie halfway between those of other similar ones. His studies focused on the atomic mass of elements, and he recognised that elements with similar properties could be bracketed together in groups of three. He referred to these as ‘triads’, essentially developing the concept of chemical ‘families’ and believed that predictions could be made on this basis.
For example, with what he referred to as the salt-forming elements (the halogens), he identified that the properties, including density, of bromine (a liquid) fell roughly halfway between those of chlorine (a gas) and iodine (a solid). Looking at the atomic masses of these elements, he determined that the atomic mass of the second (middle) element in that triad, bromine (atomic mass 80), was quite close to the arithmetic mean of the atomic masses of the first and third elements (chlorine, atomic mass 35.5, and iodine, atomic mass 127).
Döbereiner’s triadic predictions held true for iron, cobalt, and nickel, and for sulphur, selenium, and tellurium. This success was repeated for the alkaline earth metals, calcium, strontium, and barium, and the alkali metals lithium, sodium, and potassium. His work to arrange the elements according to their atomic mass, along with his classification of elements into triads was a major stride in the journey towards the modern Periodic Table.
Next came scientists like Alexandre Béguyer de Chancourtois, a French geologist, and British chemist John Alexander Reina Newlands. Both men, working independently but around the same time, created their own versions of periodic tables. Both men again, like Döbereiner, had recognised that certain properties recurred at regular intervals, echoing the periodicity displayed in the rows of the modern Periodic Table.
In 1862, de Chancourtois arranged the chemical elements in 3D graphical form. The elements were aligned in order of atomic weights spiralling around a cylinder, where one complete rotation tallied with an increase of sixteen in atomic weight. His ‘vis tellurique’ allowed similar elements to line up vertically above or below one another on the cylinder. Some believe that he so named this graph as the element tellurium fell at its centre. On the other hand, most elements are extracted as oxides, their ‘earths’ (the Latin being tellus, or telluris), a fact that would have been a significant point to a geologist.
Newlands divided all of the known elements into seven groups of eight, calling this his ‘law of octaves’. This was a nod to a musical scale, where the note C repeats at every eighth note in the C major scale. In the same way, he published in 1865 that “any given element will exhibit analogous behaviour to the eighth element following it in the table”. His version of the table showed ‘groups’ of elements horizontally, with the ‘periods’ vertically. This is the other way around to the modern form of the periodic table.
Almost concurrently, scientists across Europe were working on the same problem. The race was on to devise a system to logically classify the elements according to their atomic weight.
Images from Wikipedia, all public domain
German chemist Julius Lothar Meyer was one of these, and he developed a version of the periodic table. Again, this reflected the periodicity displayed by elements when arranged in order by atomic weight. His original table included twenty-eight of the more than sixty elements known at the time. Meyer’s table was unique, as he classified these elements into six families by their valency (how many other atoms an atom of a particular element can combine with). He published this initial version in his 1864 book Die modernen Theorien der Chemie, but his work continued. In 1870 he published his classic paper, updating the table to include all elements known at that time, Die Natur der chemischen Elemente als Function ihrer Atomgewichte.
But unbeknownst to him, Dmitri Ivanovich Mendeleev, another young man was studiously applying himself to the same problem. Like Meyer, Mendeleev had also studied under the father of spectroscopy, Robert Wilhelm Eberhard Bunsen (yes, that Bunsen, of the burners) and had worked at the University of Heidelberg, but neither was aware of the other’s interests.
Now, the Periodic Table that we are most accustomed to seeing was developed by the Siberian born Russian chemist, Mendeleev. He too had been successful in placing all of the known elements into one table. However, this was first published in 1869, thus preceding poor Meyer’s updated efforts.
A fascinating man, one story has it that Mendeleev developed his table after playing a sort of ‘chemical solitaire’ card game. He came up with this to construct a system where the sixty-three known elements fitted in relationship to each other, leaving gaps where he predicted that as yet unknown elements would sit. He claimed that the breakthrough finally came to him in a dream. When tiring of this task, he fell asleep with atomic numbers and symbols whirling through his head. Once he had woken, he realised that that the elements would be more efficiently arranged, not by their atomic numbers, but by their properties. He’s said to have remarked that:
“I saw in a dream a table where all elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper, only in one place did a correction later seem necessary.”

Pixel 17, licensed under CC BY-SA 2.0
There are many tributes to Mendeleev and his periodic table, including a station, ‘Mendeleyevskaya’, on the Moscow Metro. There is also, as one might imagine, an element named for the great man. This is a synthetic element (not naturally occurring) called Mendelevium (symbol Md). It was originally given the symbol Mv but this was, for reasons I cannot identify, amended in 1957. One of the actinides, a radioactive metal, Mendelevium is a heavy element with atomic number 101.
Perhaps my favourite monument is in Bratislava, Slovakia. Seen below, this stands outside the Faculty of Chemical and Food Technology of the Slovak University of Technology.

mmmdirt, licensed under CC BY-SA 2.0
Suffice it to say, that the story of the Periodic Table doesn’t end with Mendeleev, nor is the rectangular ‘grid’ table we usually see is not the only possible design.
In the 1960s, German chemist Otto Theodor Benfey devised a ‘table’ in the form of the Periodic Snail, spiralling out in two-dimensions from hydrogen at the centre. This extended periodic table allowed the lanthanides (rare earth elements) and actinides (radioactive metallic elements) to appear in their actual positions, rather than something which looks like a bit of an afterthought seen at the base of the standard grid tables. Benfey published an impressive article on this, in the American Chemical Society’s Bulletin for the History of Chemistry.
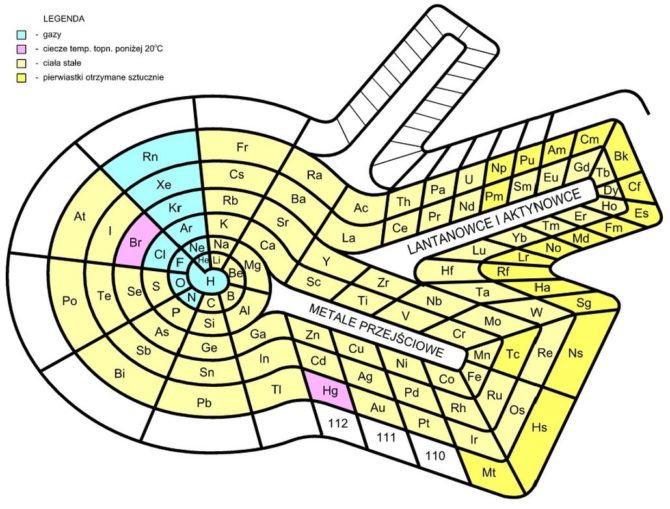
Bastianow, licensed under CC BY-SA 4.0
There are thousands of periodic tables out there in all shapes and sizes, 2D ones, or 3D, plain, colourful, some sane, some seriously kooky (Khipu or Talking Knot… I’m looking at you). There are Periodic Tables of Alchemical Symbols, with haiku written for each element, and a little bizarrely, Periodic Tables of Beer. The Table has been made in Braille, out of cupcakes, and has been applied to mugs, t-shirts, buses, even elephants. For those of you who might like to look further, a dedicated PTophile called Mark R. Leach has created an amazing online database of periodic tables & periodic system formulations.
In the next episode of this slightly rough and ready series, I’ll take a look at the element hydrogen (Ed My bad!). But for now, I intend to concentrate my efforts on this pair. Cheers, all…

Science Activism Beryllium and Erbium, licensed under CC BY 2.0
© SharpieType301 2023



